Gain new perspectives for faster progress directly to your inbox.

With the recent news that Elon Musk’s Neuralink is approved to begin human trials, it’s clear that bioelectronics like brain-computer interfaces (BCIs) are moving out of the realm of science fiction and into reality. What is a brain-computer interface (BCI)? It’s a brain implant that allows users to control a computer or external device with just their thoughts.
Neuralink involves an implant with over 1,000 electrodes on 64 threads, each thinner than a human hair, that record and transmit patients’ thoughts to an app. The app then decodes the brain signals and controls a computer, clicking on things as if using a mouse or creating text as if the user were typing. This allows patients with paralysis or neurodegenerative diseases such as Parkinson’s or amyotrophic lateral sclerosis (ALS) to communicate. Its applications go beyond mere rehabilitation; Neuralink envisions a future where thought becomes the ultimate interface, empowering people to control devices, navigate virtual worlds, and even augment their cognitive abilities.
Neuralink isn’t alone in this quest to connect brains and computers — companies like Synchron and Precision Neuroscience have also begun in-human clinical trials of BCI technologies. Many of these human trials are seeking patients with paralysis or ALS, but the opportunities for medical advances extend far beyond these diseases.
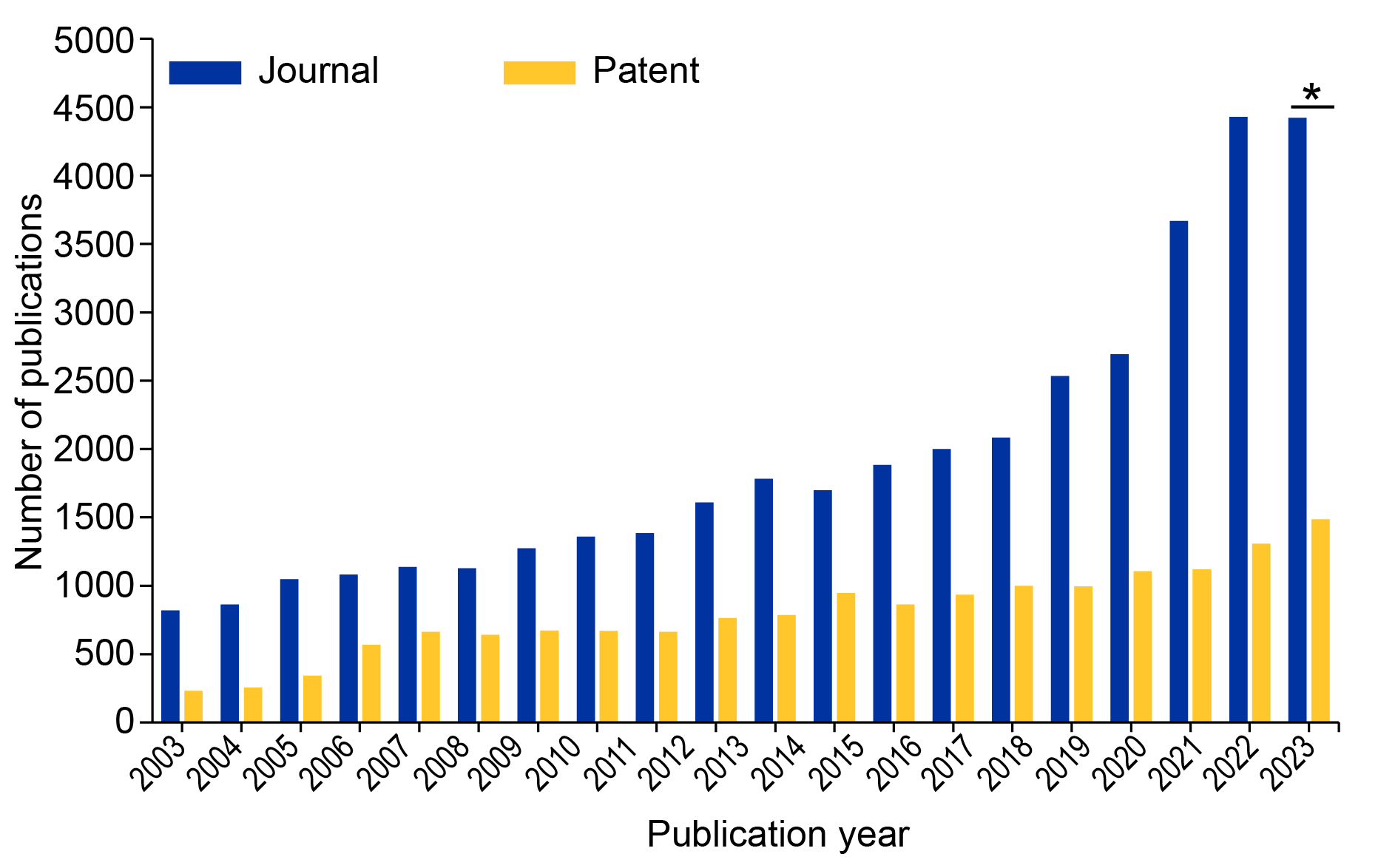
An analysis of patent and journal documents in the CAS Content CollectionTM showed a growing interest in bioelectronics, but it also revealed important challenges to its widespread use. We’ve seen substantial growth in journal publications relating to bioelectronics over the last five years, but academic research is outpacing patent publications (see Figure 1). This suggests that large-scale commercialization of these technologies faces fundamental scientific challenges that are being studied at the academic level and may be years away.
Why is it still challenging to integrate bioelectronics seamlessly with everyday life? What are the materials that make these innovations possible, and how must they improve? Our analysis of the latest research revealed several key trends.
An introduction to bioelectronics
Bioelectronics involve the development of new devices, often using novel materials and methodologies, that allow electronic systems to interface with biological components at the molecular, cellular, and organ levels. This approach capitalizes on the intrinsic ability of living organisms to sense, process, and respond to external stimuli in combination with the precision and speed of modern electronic components. To make these human-computer interfaces, these innovations rely heavily on an array of specialized materials that enable effective integration with biological tissues.
Neuralink, as mentioned, uses tiny electrodes on threads that are one-fifth the thickness of a human hair, combining conductive metals with polyamide, a type of plastic. Precision Neuroscience also utilizes thousands of minuscule electrodes embedded in a flexible film that conforms to the surface of the brain. The miniaturization of materials used in the electrodes is a unique challenge because they must have the ability to efficiently transfer electrical charge to biological tissues while maintaining softness, flexibility, and biocompatibility.
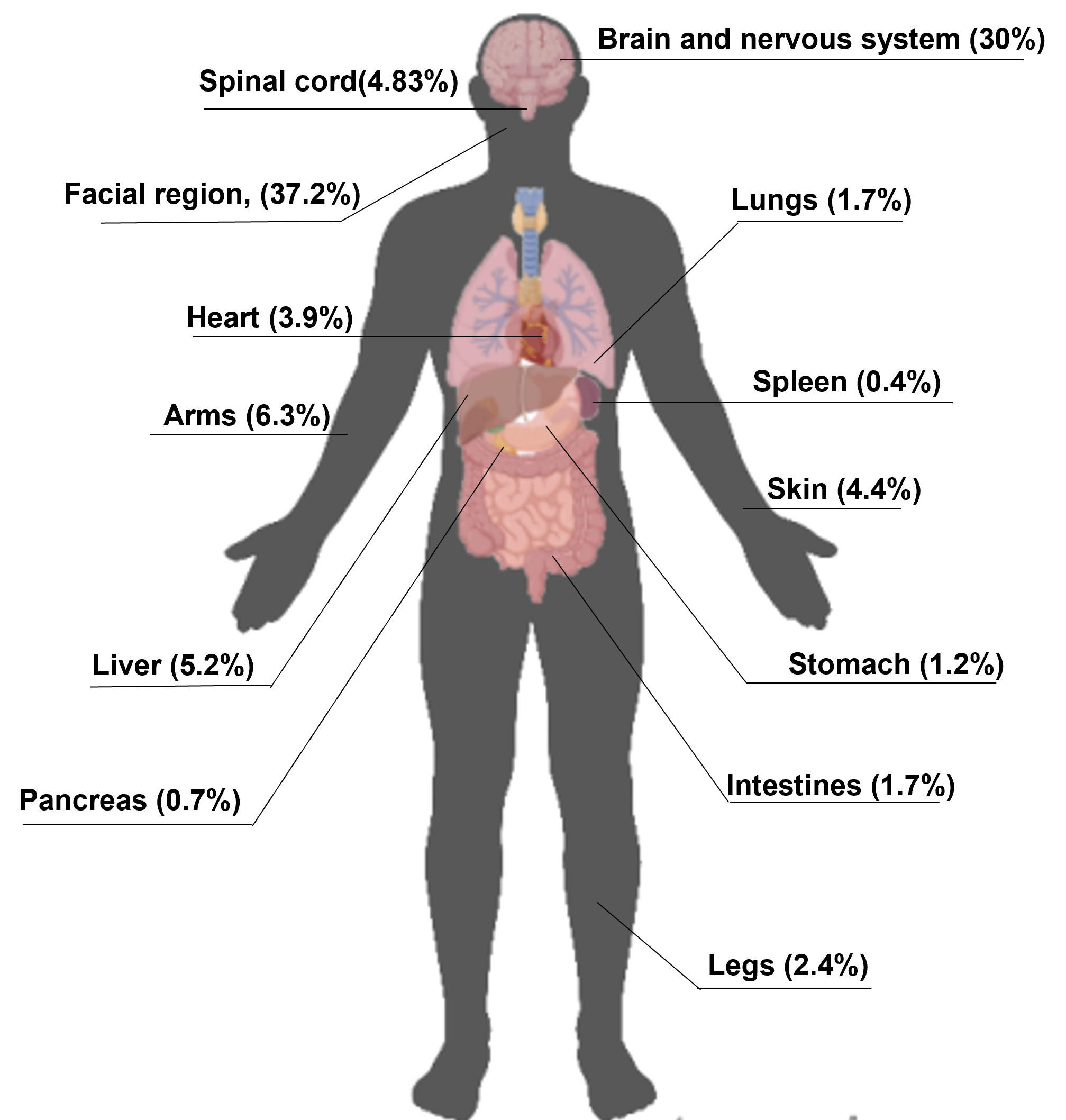
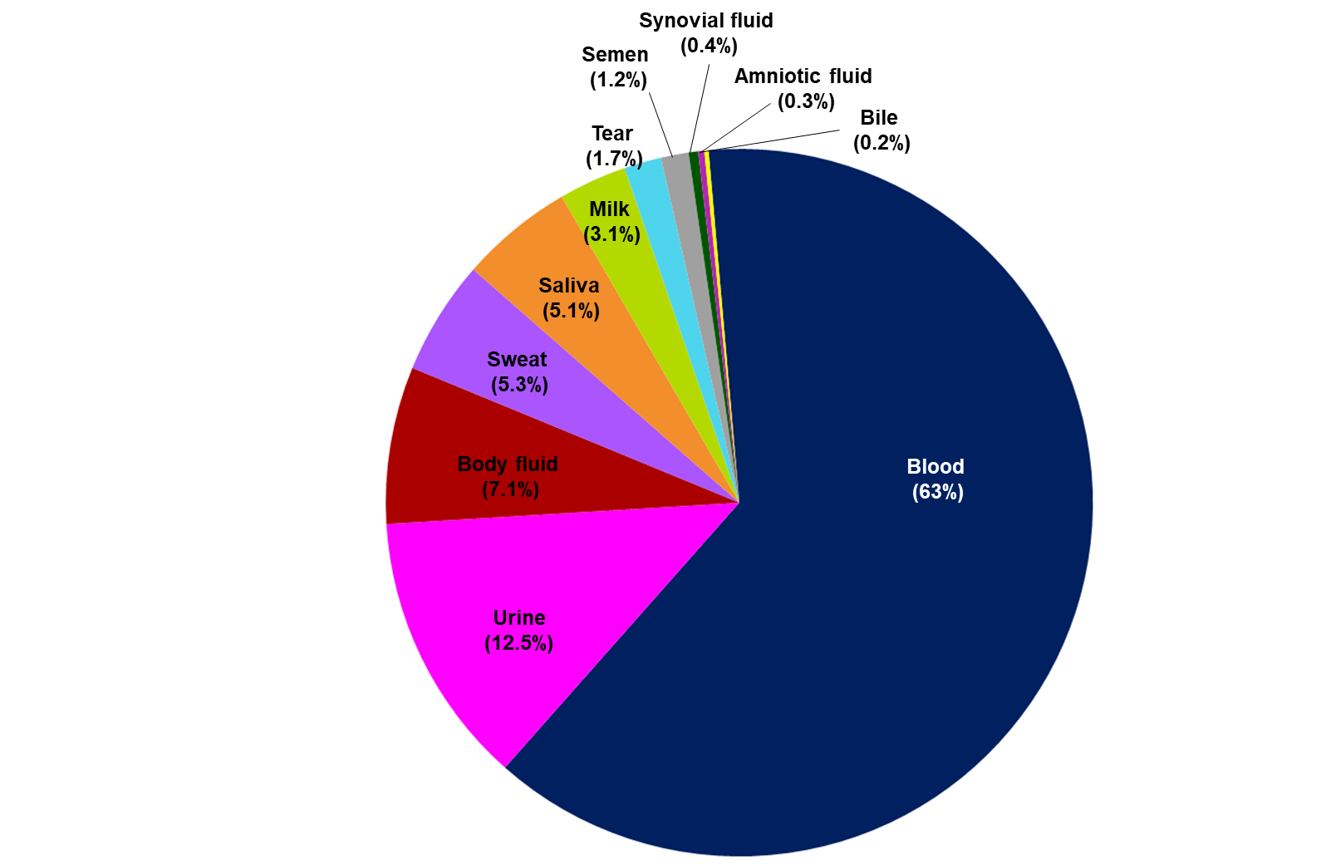
Our research showed that the facial area and nervous system are the most cited bodily areas in bioelectronics research, but nearly every system is represented to some degree (see Figure 2).
We’re seeing bioelectronics applications for cardiac monitoring, drug delivery, pain management, and more. Like BCIs, these innovations must use materials that can meet multiple demands. Those materials are detailed below.
Polymers used in bioelectronics
Polymers are molecules consisting of many units bonded together. In bioelectronics, polymer-containing composites are increasingly researched because they can bring together materials with multiple complementary properties or enable a material to function optimally for a certain application.
For example, polydimethylsiloxane (PDMS) is the most common polymer cited in bioelectronics references because of its many advantageous properties. First, its tunable flexibility and elasticity enable the creation of flexible and stretchable electronic devices that can mechanically integrate with biological tissues. PDMS-based substrates are commonly used for the fabrication of flexible electrodes, sensors, and wearable devices. PDMS is also suitable for optical applications, and PDMS-sheathed neural interfaces can be implanted in the brain or nervous system without significant tissue damage or provoking an immune response.
Carbon nanotubes are a type of conductive material that can be combined with PDMS. These structures, typically 10s of nm in diameter, provide electrical conductivity and can be mixed with PDMS to be used across biomedical applications, like cardiac muscles and the brain.
Another promising material is PEDOT:PSS, a combination of polymers with ideal conductive and mechanical properties. This material can be used in hydrogels, a class of materials with properties similar to human tissues that are appropriate for bioelectronic applications. For example, a recent patent describes an injectable gel electrode containing PEDOT:PSS that is sent to a tumor resection cavity to deliver electrotherapy treatments targeting residual cancer cells post-surgery.
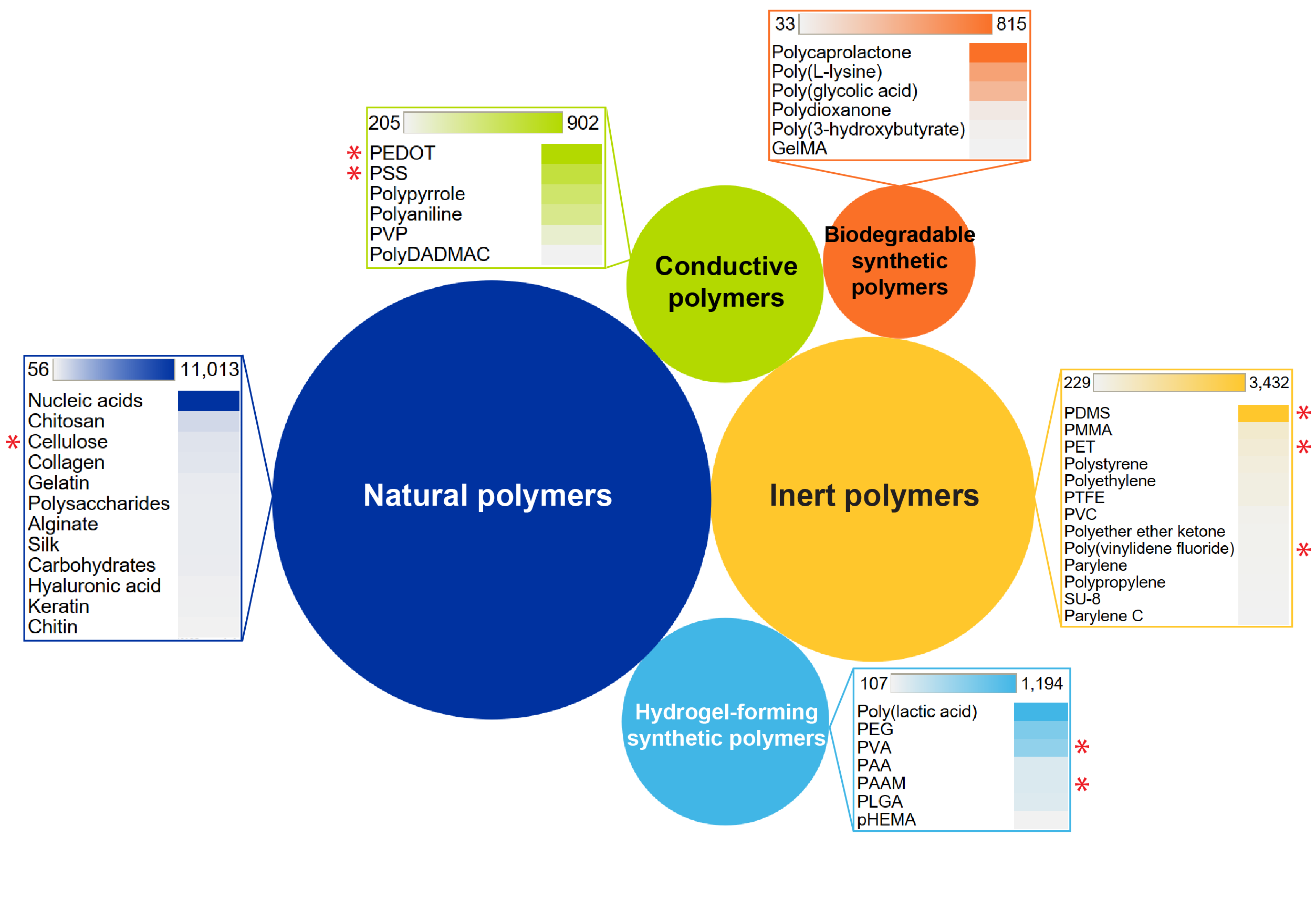
These synthetic polymers and microscopic materials like nanotubes are promising, but we also found that scientists haven’t forgotten about materials from the natural world that can be combined to make bioelectronic composites (see Figure 3).
Natural polymers, including cellulose, alginate, and silk, have the advantages of stability, high mechanical strength, and biocompatibility. Silk-based electrodes combined with PEDOT:PSS demonstrated excellent stretchability and comfort for wearable devices. Another recent innovation is a combination of biodegradable molybdenum oxide and sodium alginate — developed from brown algae — that makes a resorbable supercapacitor to power implanted bioelectronic devices.
Metals and inorganic compounds in bioelectronics
That brings us to another class of materials — metals. Noble metals are conductive and stable, so they have featured prominently in bioelectronics research. Neuralink, for example, uses gold and platinum, two highly stable and well-understood metals.
Our analysis, however, revealed that biodegradable and bioresorbable metals like molybdenum, zinc, and magnesium are also promising inorganic compounds for bioelectronics. These materials combine the electrical properties of a metal with the ability to be safely broken down and absorbed by the body over time. Fully resorbable bioelectronic devices have been constructed by combining these metals with biodegradable encapsulating polymers, such as an electronic nerve stimulator electrode consisting of molybdenum and polyurethane.
Overcoming hurdles to commercialization
There are numerous innovative possibilities with bioelectronics, and our journal and patent research showed that these examples discussed above are just a handful of the potential use cases. However, this field is not without significant challenges, and most of this technology is still experimental.
One of the key challenges is the immune response to these materials and devices. Every patient is different, and it will take extensive trials and research to demonstrate that individual bioelectronic materials are safe for widespread use. Carbon nanotubes, for example, can have a range of structures, making it even more difficult to predict how each person will react. BCIs are implanted in sensitive brain tissue and may even have cybersecurity implications in the future.
The only path forward to commercialization is the clinical trial process and in vivo testing. Long-term effects must be studied to determine toxicity and effectiveness — do the conductive materials maintain their ability to effectively transmit electrical charge? Will these polymers break down inside the body? Are there long-term implications of BCI implants on human physiology or psychology?
Addressing these questions is an important challenge, but what’s also important is the potential of these innovations to improve quality of life. We’re excited to see how the research evolves in the coming years as human trials are ramped up, and more concepts reach the patent application stage. It may still feel like science fiction, but bioelectronics will be a key component of future medical care. To learn more about how bioelectronics and other emerging classes of biomaterials will reshape the future, view our report on the emerging trends in biomaterials.
This article incorporates research completed in collaboration with Westlake University, China.