Gain new perspectives for faster progress directly to your inbox.
Alzheimer’s disease is a progressive neurological disorder with devastating effects for individuals and families. According to the Alzheimer’s Association, nearly 7 million Americans are living with the disease, and that number is projected to reach close to 13 million by 2050. The World Health Organization estimates that 50 million people worldwide are suffering from a form of dementia, with Alzheimer's accounting for up to 70% of cases.
Currently, there is no specific test to diagnose Alzheimer's, and despite extensive research, there is still no cure. The scientific community is aware of the immense challenges it presents, and as our analysis of the CAS Content CollectionTM shows, promising developments are taking place in several areas of related research, particularly disease identification via biomarkers.
Reliable biomarkers, the measurable indicators or characteristics that can provide information about the presence, progression, or risk of developing a disease, are important for any condition. Identifying biomarkers and gaining a better understanding of the pathophysiology of Alzheimer's will drive drug development to target the cause rather than the symptoms. New research indicates that breakthroughs in biomarkers, personalized medicine, and new treatments may be on the horizon, providing hope for tackling this devastating disease.
Causes of Alzheimer’s disease
Alzheimer's is associated with abnormal protein deposits in the brain, specifically beta-amyloid plaques and tau tangles. The accumulation of these plaques and proteins disrupts normal neuronal function, leading to dysfunction and impaired neurotransmission. As the disease progresses, neurons become increasingly vulnerable and eventually undergo cell death, resulting in widespread neuronal loss, particularly in brain regions critical for memory and cognitive function, such as the hippocampus and cerebral cortex.
The causes of Alzheimer's are varied and include advanced age, genetic factors, and environmental and lifestyle influences. Advanced age is the primary risk factor — the number of cases doubles every five years for people beyond age 65 — and as life expectancy increases worldwide and more people undergo the effects of aging, cases are expected to grow significantly.
However, aging itself is not the only factor. Obesity, cardiovascular disease, social isolation, and a lack of mentally stimulating activities have all been linked to the disease. Genetics are critical as well — familial Alzheimer's is a small percentage of cases but it’s an autosomal dominant pattern with mutations in the Presenilin-1 (PSEN-1) and Presenilin-2 (PSEN-2) genes. In late-onset Alzheimer's, by far the most common form of the disease, mutations to Apolipoprotein E (ApoE), Clusterin (CLU), and Bridging Instigator 1 (BIN1) genes are risk factors. Identifying these and other genes that play a role in the disease's development is a crucial step to finding treatment breakthroughs.
Research trends relating to Alzheimer’s disease
To better understand the Alzheimer's research landscape, we analyzed the CAS Content Collection, the largest human-curated collection of published scientific information. Currently, there are over 300,000 scientific publications relating to the disease, with over 250,000 journal articles and nearly 50,000 patents worldwide. There has been steady growth in documents over the last three decades, with a greater than 30% increase in just the last three years (see Figure 1).
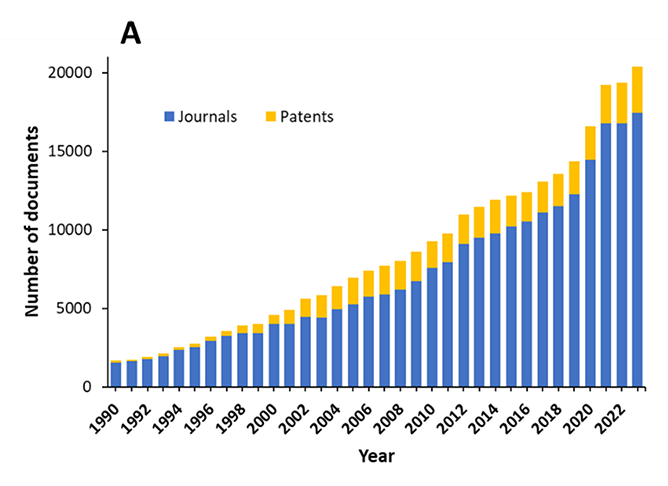
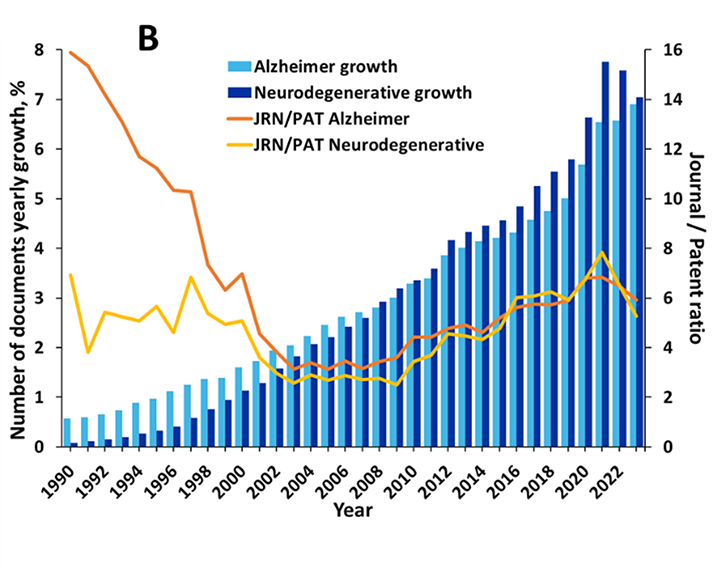
The prevalence of journal articles over patents suggests that research remains the main area of emphasis, rather than the commercialization of therapies. As seen in Figure 2, aging and β-amyloid are the most widely explored concepts in the literature because these are the leading risk factor and the leading physiological cause of Alzheimer's respectively.
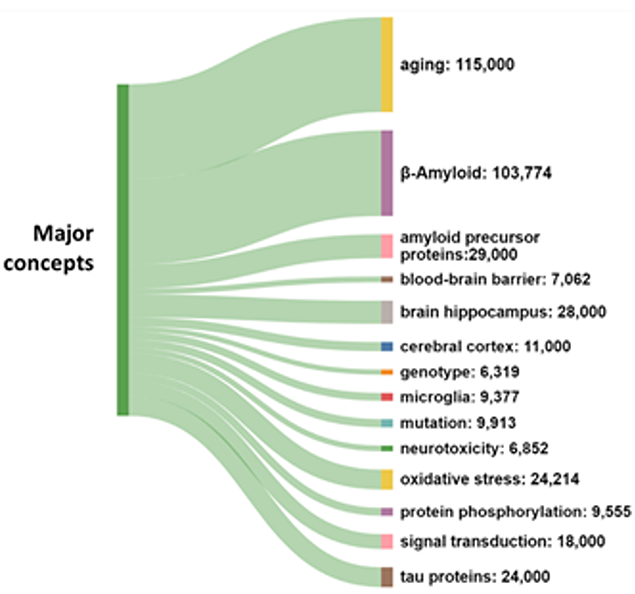
Biomarkers are the fastest-growing concept in Alzheimer’s research
In clinical practice today, Alzheimer's biomarkers include amyloid peptides and phosphorylated tau, which are drawn from cerebrospinal fluid (CSF) and blood plasma. The procedure for detecting these biomarkers is highly invasive, so researchers are pursuing less invasive and more cost-effective options, particularly blood-based biomarkers.
For example, amyloid beta, tau, and biomarkers of neuronal injury and degeneration, such as the neurofilament light chain, can indicate the presence of Alzheimer's. Neuroinflammation biomarkers are also being investigated for their potential role in pathogenesis and progression (see Figure 3). Neuroinflammation is a major complication in Alzheimer's and causes many symptoms, so identifying this inflammation and treating it can alleviate these issues.
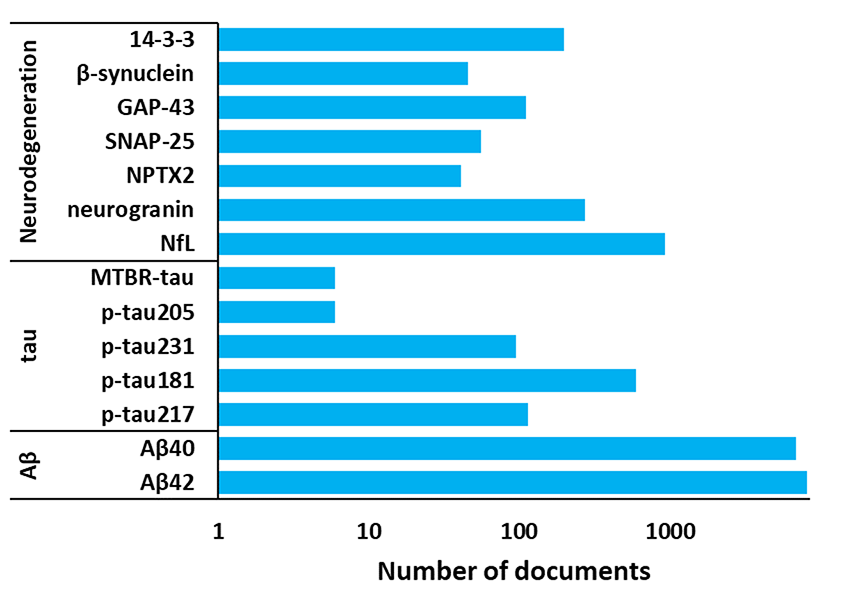
Genetic biomarkers are increasingly important for risk identification, namely the genes PSEN-1 and PSEN-2 for familial Alzheimer's risk, and ApoE for late-onset Alzheimer's risks. Mutations on the Amyloid Precursor Protein (APP) gene located on chromosome 21 have also been found to play a key role in disease development, namely by leading to increased production or altered processing of beta-amyloid, resulting in the accumulation of amyloid plaques in the brain.
More advanced scanning technology is also providing hope for better diagnostics — PET imaging using radiotracers that bind to amyloid plaques allows for the visualization and quantification of amyloid deposits in the brain, thereby helping to confirm the presence of Alzheimer's. MRIs can also detect structural and functional changes in the brain to identify the presence of the disease.
These research efforts are advancing our understanding of Alzheimer's biomarkers, and they have the potential to improve early diagnosis, monitor disease progression, and assess treatment responses.
Pharmacological targets and genetic risk factors
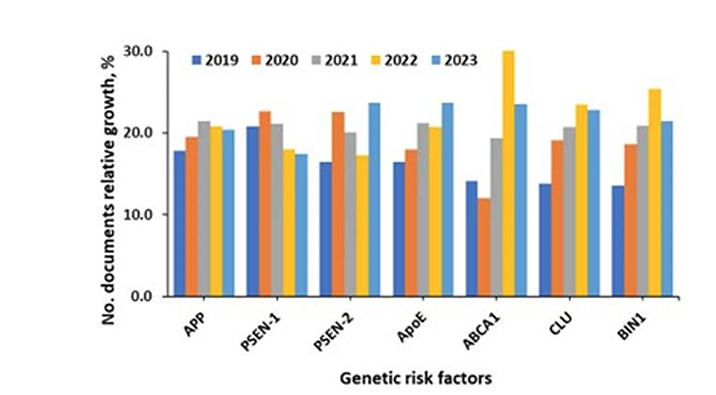
As genetic risk factors and disease pathogenesis are better understood, the correlation between these risk factors and pharmacological targets in publications becomes more important. According to our analysis of the CAS Content Collection, the largest number of documents associate APP, ApoE, PSEN-1, and CLU genes with AD pathogenesis. The fastest-growing documents are related to CLU, BIN1, ApoE, and Adenosine triphosphate-binding cassette transporter A-1 (ABCA1). This gene is known to regulate the stability of ApoE lipidation (see Figure 4).
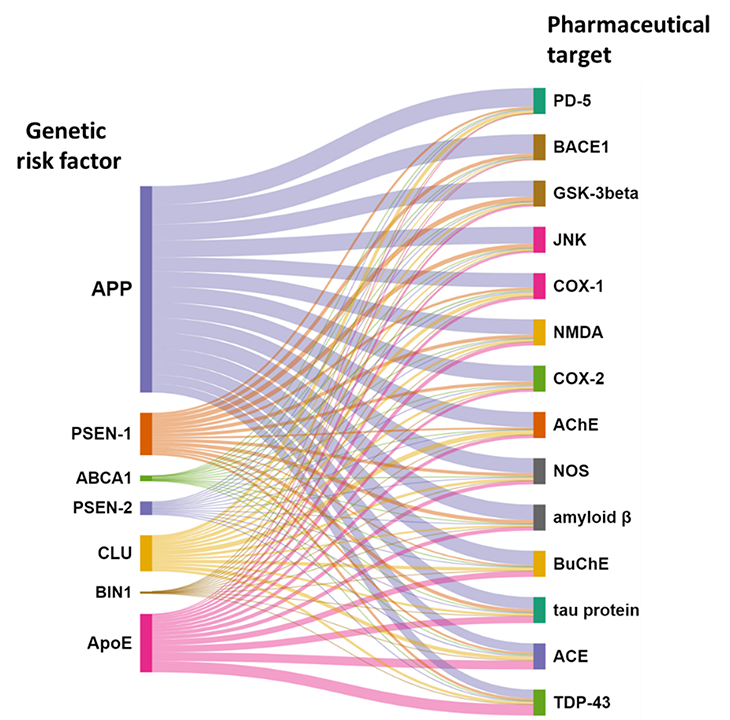
We’re beginning to see significant co-occurrences in the literature between these genetic risk factors and target proteins and enzymes (see Figure 5). This is important for several reasons:
- By identifying genetic variations that increase the risk of Alzheimer's, researchers can potentially tailor treatments to the relevant pharmacological pathway, leading to more effective therapies and ideally fewer side effects.
- Understanding the genes associated with Alzheimer's pathology can drive the development of drugs that intervene in those pathways.
- If certain genetic markers are found to be strong predictors of Alzheimer's, individuals with those markers could be monitored more closely and potentially receive preventive care before symptoms emerge.
- Studying how genetic variations influence the drug effectiveness can provide valuable insights on the underlying mechanisms of Alzheimer's and more effective treatment strategies.
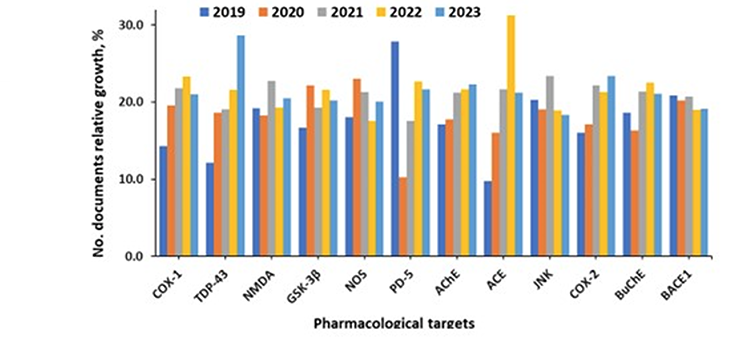
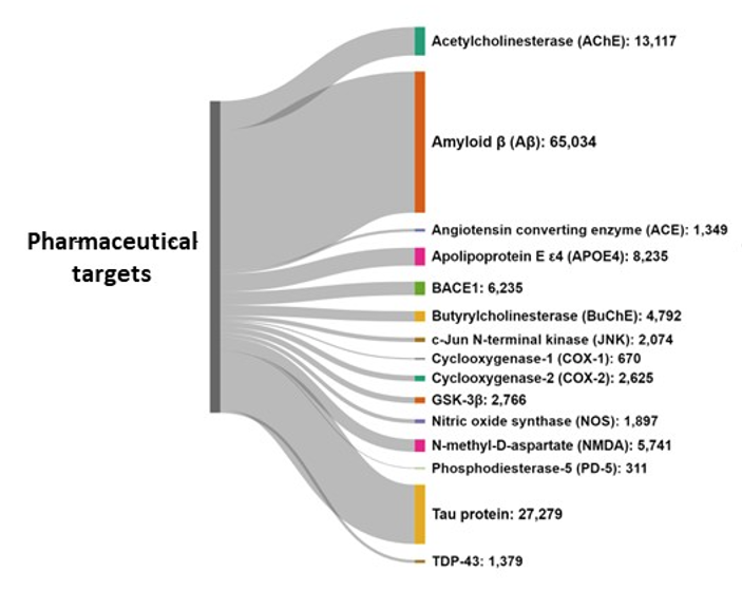
Amyloid-beta and tau proteins are the undisputed major pharmacological targets, while the fastest-growing targets being researched are TDP-43, a nuclear protein involved in the regulation of gene expression, and Angiotensin-converting enzyme (ACE) (see Figure 6).
TDP-43 aggregates appear to be toxic to neurons, and they’re found in a large percentage of Alzheimer's patients. There is also emerging evidence that TDP-43 may spread in a prion-like manner, propagating cell-to-cell and potentially contributing to disease progression. In response, researchers are pursuing numerous treatments relating to TDP-43: some that can prevent TDP-43 aggregates from forming, others that break up existing TDP-43 aggregates, and other potential drugs to protect neurons from the toxic effects of this protein.
Researchers are also finding that ACE may play a role in brain inflammation, a hallmark of Alzheimer's. Targeting this enzyme is still in the early stages of research, but it could be a promising avenue for drug development.
Drug development and ideas to watch
While there is no known cure for Alzheimer's, numerous drugs have been approved to slow its progress, and new possibilities are in the pipeline. Cholinesterase inhibitors, for example, are prescribed to treat symptoms associated with memory, thinking, language, and judgment. Current drugs on the market include Donepezil, known as Aricept®.
Glutamate regulators are another existing drug class prescribed to improve memory, attention, thinking, language, and the ability to perform simple tasks. Glutamate regulators such as Memantine (Namenda®) can also be used with cholinesterase inhibitors as a combination therapy.
However, the most exciting advances in drug development involve targeting Alzheimer's pathophysiology rather than its symptoms. Drugs in development are focusing on amyloid beta and tau protein tangles, which could mark game-changing developments in treating the causes. Only one of these anti-amyloid antibody drugs is currently approved (Lecanemab), but more are in Phase III clinical trials (see Table 1).
Several monoclonal anti-tau antibodies are also in Phase II trials, which may or may not show efficacy, but they do signal new directions in drug development. These treatments could offer hope as disease-modifying treatments, not just drugs to delay or mitigate symptoms.
|
Drug name |
CAS RN® |
Structure |
Drug type |
Clinical trial # |
|
Phase 3 |
||||
|
Gantenerumab |
1043556-46-2 |
Protein/peptide sequence |
Anti-amyloid antibody |
NCT01760005 |
|
CAD106 (Amilomotide) |
1176290-10-0 |
N/A |
Amyloid vaccine |
NCT02565511 |
|
TRx0237 (LMTX, Methotrexate) |
59-05-2 |
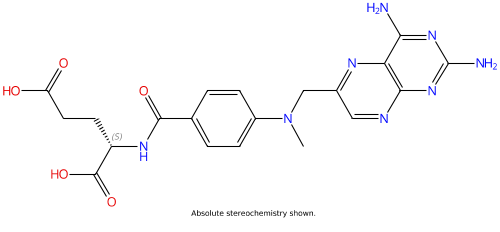 |
Tau protein aggregation inhibitor |
NCT02245568 |
|
AGB101 (Levetiracetam) |
102767-28-2 |
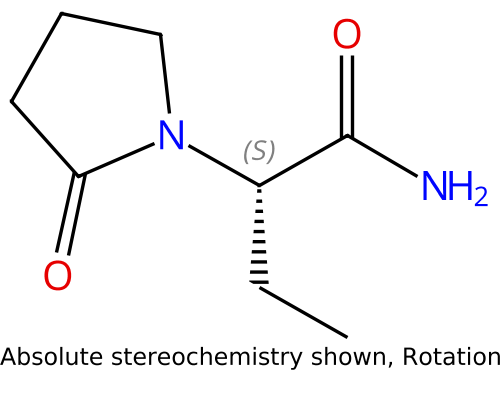 |
Low-dose levetiracetam - improves synaptic function and reduces amyloid-induced neuronal hyperactivity |
NCT05986721 |
|
ALZT-OP 1 (cromolyn + ibuprofen) |
2396743-42-1 |
N/A |
Combination treatment (mast cell stabilizer + NSAID) |
NCT02547818 |
|
Azeliragon |
603148-36-3 |
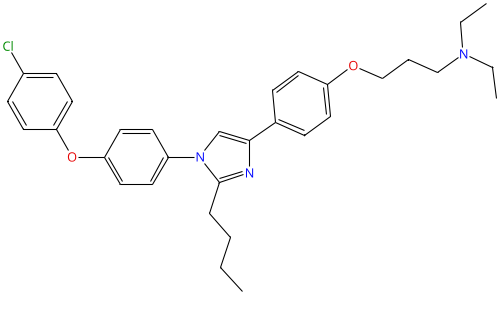 |
Small-molecule RAGE inhibitor |
NCT02080364 |
|
BHV4157 (troriluzole) |
1926203-09-9 |
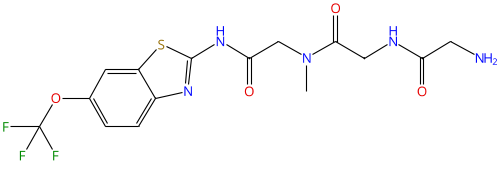 |
Glutamate modulator |
NCT03605667 |
|
Masitinib |
790299-79-5 |
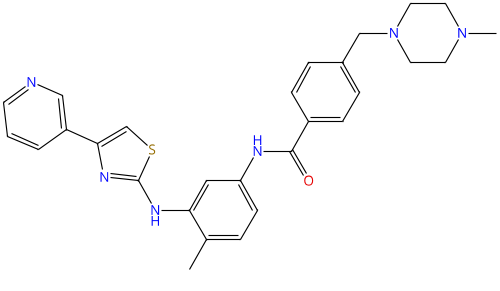 |
Tyrosine kinase inhibitor |
NCT05564169 |
|
Remternetug |
2571940-41-3 |
Protein/peptide sequence |
Anti-amyloid antibody |
NCT05463731 |
|
Simufilam |
1224591-33-6 |
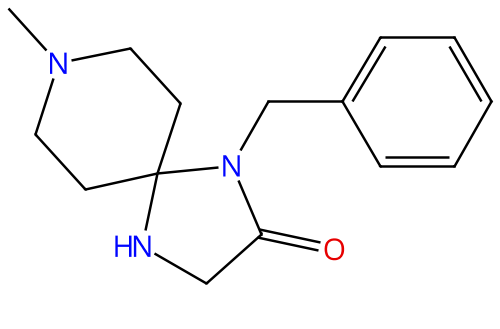 |
Stabilizing a critical protein in the brain |
NCT04994483 |
|
Solanezumab |
955085-14-0 |
Protein/peptide sequence |
Anti-amyloid antibody |
NCT00905372 ; NCT00904683 |
|
Valiltramiprosate (ALZ-801) |
1034190-08-3 |
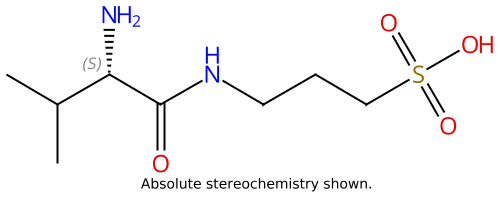 |
Beta-amyloid inhibitor, oral prodrug of the active agent tramiprosate |
NCT04770220 |
Table 1: Exemplary ongoing clinical trials phase 3 of anti-Alzheimer's therapy.
Future directions for Alzheimer’s research
Alzheimer's remains one of the most challenging diseases, and the number of patients and families affected will increase as global life expectancy does. However, as our research shows, there are potential breakthroughs on the horizon in many Alzheimer's-specific fields:
- Identifying biomarkers for early detection, accurate diagnosis, and monitoring of disease progression
- Drug development, including drug repurposing and monoclonal antibodies
- Genetics and personalized medicine to tailor treatments and interventions to the specific genetic and biological characteristics of each individual, potentially improving treatment efficacy and minimizing side effects
- Research in non-pharmacological interventions, such as cognitive training, physical exercise, diet, and lifestyle modifications to reduce risk factors
The Alzheimer's research landscape is robust, and there is potential for meaningful breakthroughs in biomarkers, genetics, and beyond. With continued effort and investment, we can hopefully change the narrative around this debilitating disease.
Key data in this article, as well as Figures 1-6, are extracted from Tenchov R, Sasso JM, Zhou QA. Polyglutamine (PolyQ) Diseases: Navigating the Landscape of Neurodegeneration. ChemRxiv. 2024; doi:10.26434/chemrxiv-2024-pt7wp.