Gain new perspectives for faster progress directly to your inbox.

Chinese startup Betavolt recently announced it developed a nuclear battery with a 50-year lifespan. While the technology of nuclear batteries has been available since the 1950s, today’s drive to electrify and decarbonize increases the impetus to find emission-free power sources and reliable energy storage. As a result, innovations like Betavolt’s are bringing renewed focus to nuclear energy in batteries.
Nuclear batteries — those using the natural decay of radioactive material to create an electric current — have been used in space applications or remote operations such as arctic lighthouses, where changing a battery is difficult or even impossible. The Mars Science Laboratory rover, for example, uses radioisotopic power systems (RPS), which convert heat from radioactive decay into electricity via a thermoelectric generator. Betavolt’s innovation, however, is a betavoltaic battery that uses beta particles rather than heat as its energy source.
Before expecting to find these long-lasting batteries in common devices, it’s important to understand some key tradeoffs. The long lifespan of betavoltaic batteries is counterbalanced by their relatively low power output per unit mass, known as power density. The power density of the current betavoltaic batteries is so low that they cannot power a cell phone or laptop.
There are additional challenges that hinder the wider usage of these and all types of nuclear batteries, particularly material supply and discomfort with the use of radioactive materials. Yet, the physical and materials science behind this technology could unlock important advances for CO2-free energy and provide power for applications where currently available energy storage technologies are insufficient.
How do betavoltaic batteries work?
Betavoltaic batteries contain radioactive emitters and semiconductor absorbers. As the emitter material naturally decays, it releases beta particles, or high-speed electrons, which strike the absorber material in the battery, separating electrons from atomic nuclei in the semiconductor absorber. Separation of the resulting electron-hole pairs generates an electric current in the absorber, resulting in electrical power that can be delivered by the battery.
However, the process isn’t on the scale of a large nuclear power plant. Emitters and absorbers are thin films sandwiched together inside the batteries, which, like Betavolt’s, are the size of a coin or piece of candy. This is because the majority of beta particles are absorbed, and their energy is converted into electricity close to the absorber’s surface.
A similar process produces electric currents in solar panels with photovoltaic cells. In that application, photons from the sun dislodge electrons, forming electron-hole pairs in the absorber, whereas beta particles generated by natural radioactive decay are responsible for that process in a betavoltaic battery.
Challenges with nuclear batteries
Due to the physical limitations of the decay process and the conversion of beta particles to electricity, these batteries only produce a tiny amount of power — on the order of microwatts. As seen in Figure 1, betavoltaic batteries have very low power density, but they offer extremely high energy density, or total energy the battery contains per unit of mass, compared to other types of batteries.
Betavoltaics have such high energy density because the radioactive emitters decay slowly over time, leading to the reaction emitting electrons for many years. Their lifetime is measured by their half-life, which is the amount of time they take to reach half of their initial beta particle emission intensity. The most common emitters in betavoltaic batteries have half-lives of 2.5 to 100 years.
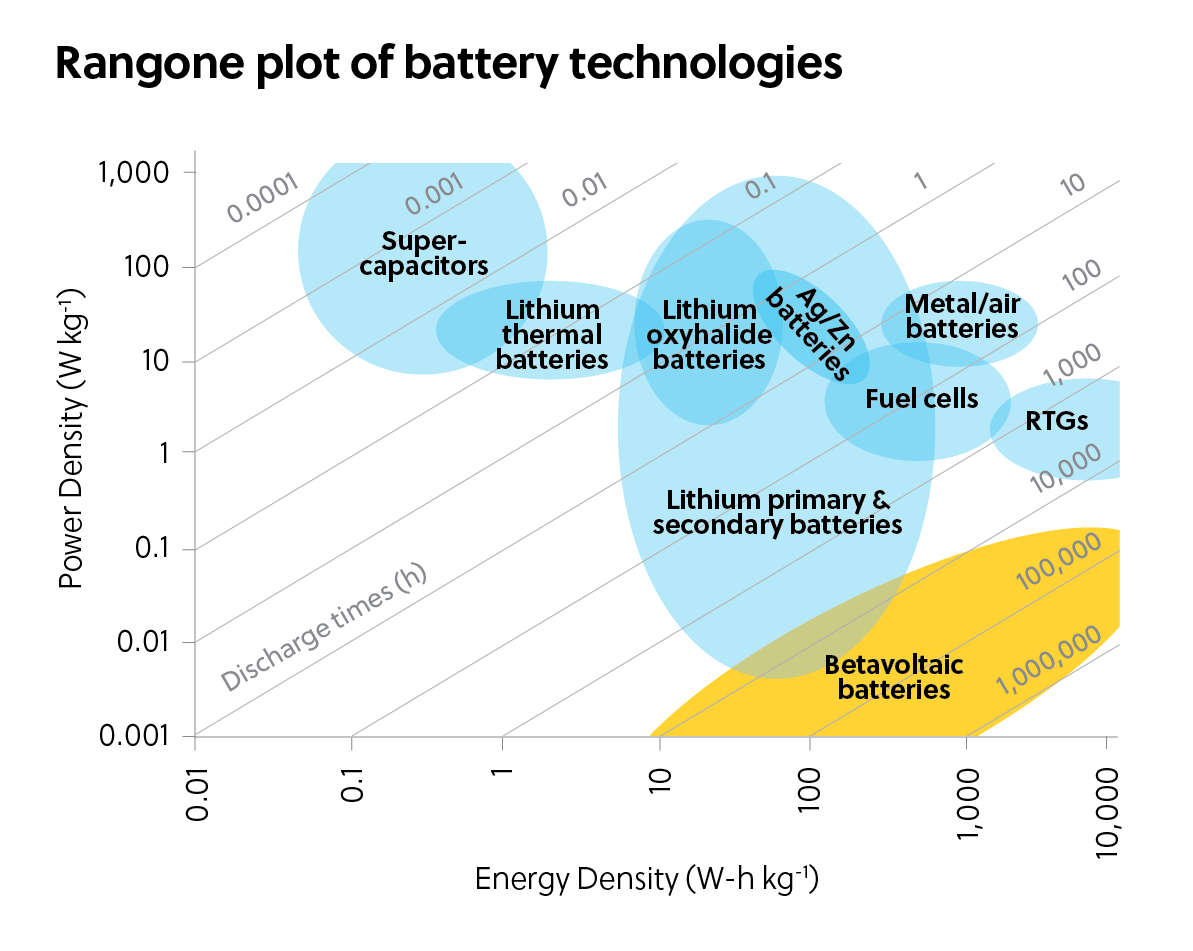
It would take extremely large stacks of betavoltaic batteries to produce watts or kilowatts of power. Building batteries of that size is cost-prohibitive with present technology. One major reason is that emitters are not made of naturally occurring substances. Rather, this radioactive material must be artificially synthesized, and the cost of developing large batteries for higher power applications is infeasible.
However, betavoltaic batteries have been used in pacemakers and other small devices. With the recent growth of wearables and smart home devices, there are more potential applications than ever, owing to the successful pairing of emitters and absorbers.
Emitters and absorbers power betavoltaic technology
Providing long-term, carbon-free energy sources is vital, and while betavoltaic batteries may not power entire buildings, understanding the relationship between materials that can generate continuous energy unlocks new possibilities. By analyzing the CAS Content CollectionTM, the world’s largest human-curated collection of published scientific information, we can identify promising materials and research trends.
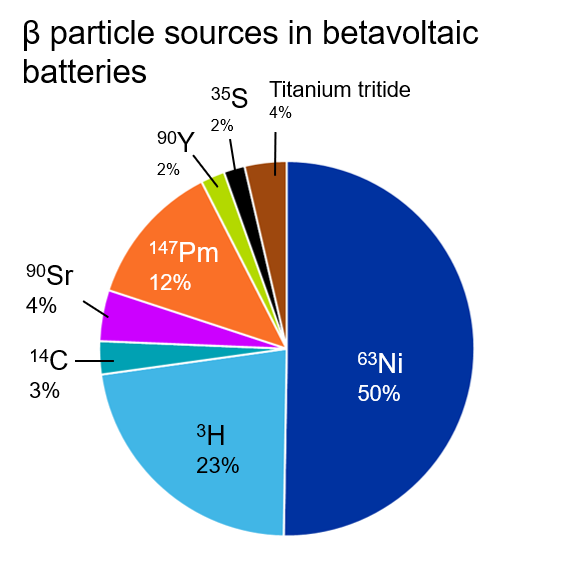
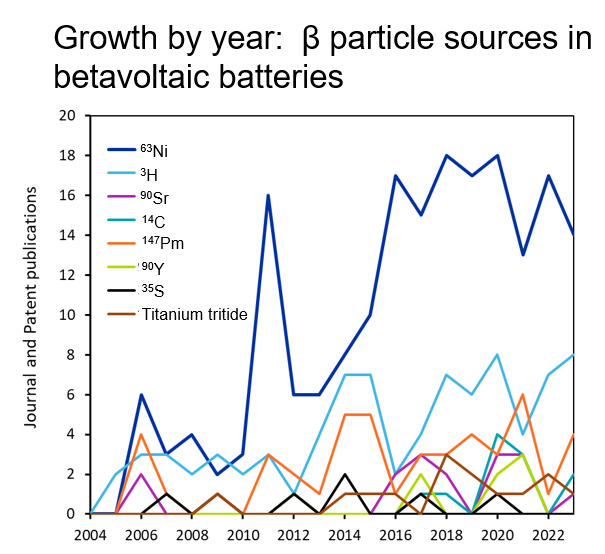
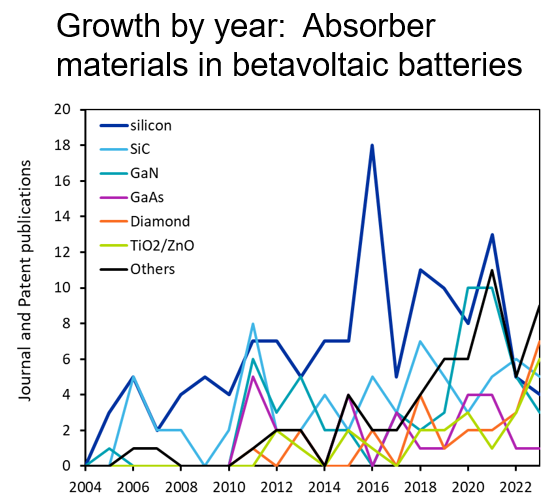
The most common beta particle emitter we see in the literature is nickel-63, an isotope of the element nickel that has a half-life of about 100 years (see Figure 2). This is followed by hydrogen-3 or tritium, which is often incorporated in the solid-state material titanium tritide. Promethium-147 appears frequently in our analysis as well, but it is less cited and has not seen the same growth as other emitter materials (Figure 3).
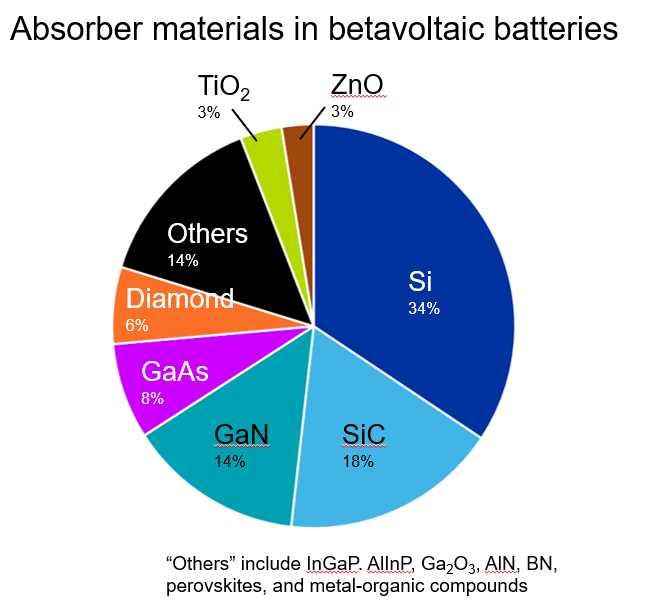
For absorbers, the most cited material is silicon, which is the most common material in semiconductor devices (see Figure 4). Silicon’s use in solar cells also demonstrates its usefulness and scalability in these types of current-generating applications.
Other frequently cited materials include silicon carbide (SiC), gallium nitride (GaN), and gallium arsenide (GaAs). These materials feature a large band gap, which increases the efficiency of the beta particle-to-electricity conversion. They also have good resistance to degradation from beta particle radiation (also known as radiation hardness), improving the lifetime and stability of the device. These are both key properties of absorber materials.
Notably, we’re also seeing diamond as a well-researched absorber material. This refers to a synthetic diamond film, not natural gems, but it is also effective as an absorber because of its large band gap and high radiation hardness.
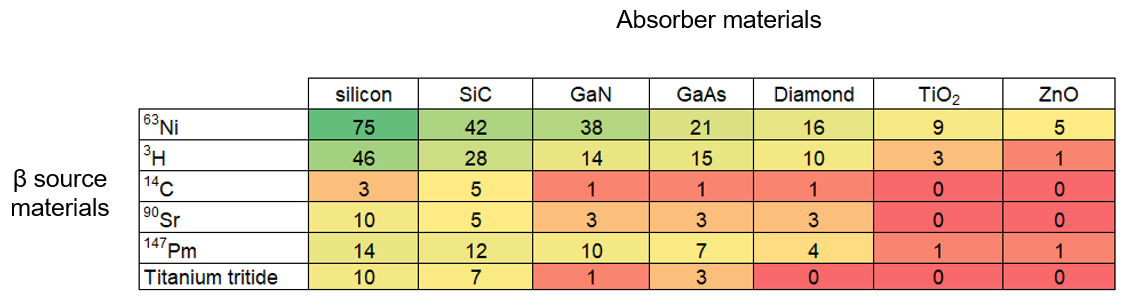
Our analysis shows that 63Ni-silicon is the most common emitter-absorber pair being researched. Beyond these materials, the frequency of emitter-absorber pairs roughly corresponds to the frequency of individual emitter and absorber materials in the literature. This suggests that researchers are currently testing many material combinations (see Figure 6).
Future applications of nuclear battery technology
If betavoltaic batteries can increase their power density while managing size and cost challenges, these batteries could power devices for many years without replacement. Because beta radiation’s penetration depth is relatively small, emitters are safer than other types of radioactive materials and can be shielded with simple materials to make them appropriate for consumer use.
Researchers in the UK have even developed a betavoltaic battery using radioactive carbon-14 from nuclear waste. They embedded the carbon-14 in the diamond to maximize efficiency, as opposed to keeping the emitter and absorber in separate layers. If produced at scale, these can help address issues with radioactive waste products while providing long-term, consistent power.
Another possible innovation is to use nanomaterials, such as carbon nanotubes or nanoporous structures, to increase the surface area of the absorbers. This would allow them to generate and efficiently separate more electron-hole pairs and hence produce a stronger current, while not increasing the size of the battery to an unsustainable degree. Increasing surface area through the use of nanomaterials has been applied to solar cells and electrochemical batteries such as lithium-ion.
If researchers can master this capability in betavoltaic batteries, it would open up this type of energy storage to even more applications and potentially lead to advances in renewable energy and energy storage to support decarbonization. To learn more about the future opportunities in green energy, see our recent article on fastest growing research trends, li-ion battery recycling breakthroughs, and key building blocks for green hydrogen economies.