Gain new perspectives for faster progress directly to your inbox.

Carbon capture and the road to Net Zero
It is paradoxical that whilst carbon dioxide (CO2) is essential for all plant life on Earth upon which all animal and human life depends, too much of this essential gas in the atmosphere is causing global warming, threatening the very survival of some populations.
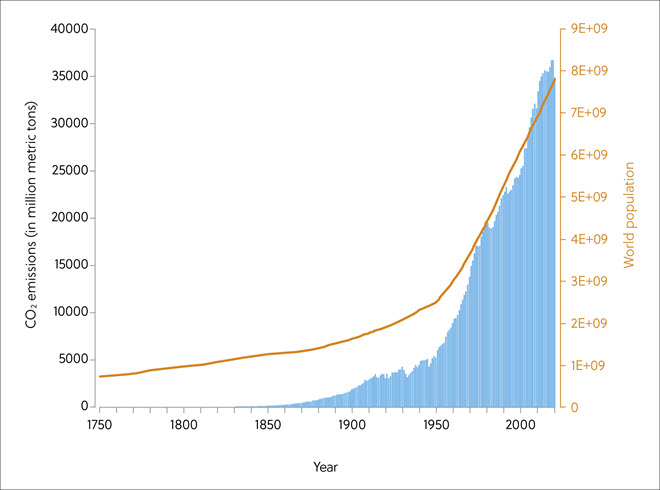
The problem of CO2 emissions from increased burning of fossil fuels dates to the 18th century, with the start of the industrial revolution in certain countries. Today, scientists have estimated the rise in average global temperatures is expected to reach 1.5oC by 2030–2052 (Figure 1). The volume of CO2 emissions is exacerbated by industrialization, urbanization, and sharply increasing world populations (Figure 2).
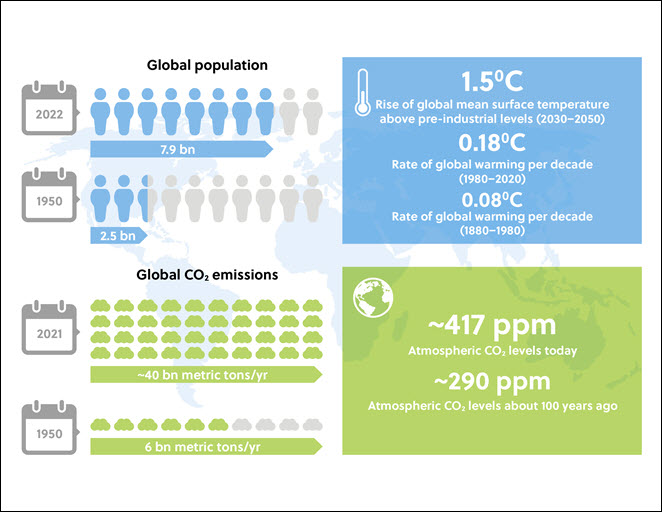
The 21st Conference of Parties on Climate Change (COP21) in 2015 adopted an ambitious target called the ‘race to zero’ by 2050. This momentous goal of eliminating net carbon emissions completely in under 30 years will require universal changes in global industrial processes and domestic energy practices. The better-known methods to achieve this goal involve various sustainable forms of power generation, such as wind and solar power; however, a less publicized but equally important approach is the capture of CO2 at the source or directly from the atmosphere (carbon capture). The technologies involved are restricted by high costs and somewhat limited storage capacity so currently, only 0.1% of global CO2 emissions are sequestered; this is predicted to rise to 19% by 2050. Research efforts in carbon capture technology have increased in recent years, but to date, only a few applications have been commercially deployed. With increasing public awareness and urgency around preventing or reducing climate change, the pressure is on to devise more efficient carbon capture technologies.
Carbon capture in the CAS Content Collection™
The CAS Content Collection™ is the largest human-curated collection of published scientific knowledge, suitable for quantitative analysis of global scientific publications against variables such as time, research area, formulation, application, and chemical composition. To assess the recent and ongoing research effort into carbon capture, an important new CAS Insight provides an overview of the latest trends. The Insight summarizes the results of an extensive recent analysis (∼18,500 documents published between 2000 and 2021) detailing terms related to carbon capture, including methods employed, storage or conversion, which were used in combination with terms related to atmospheric CO2 or its environmental effect.
Key research trends and carbon capture methods
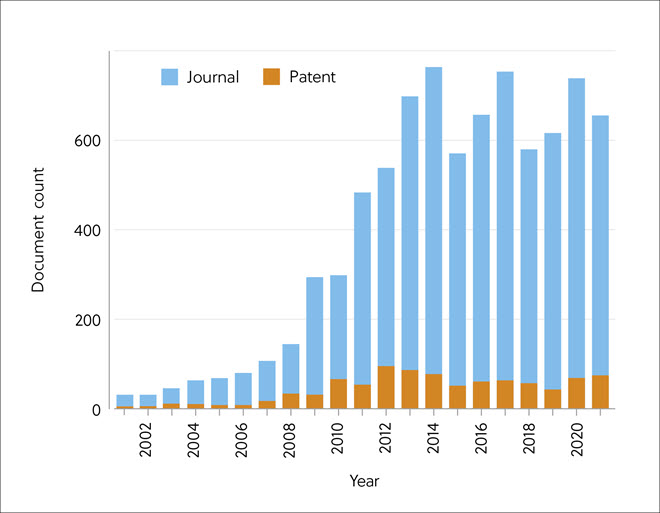
The literature analysis revealed that since 2008, there was a rapid increase in all carbon capture and storage publications, which slowed after the mid-2010s, but more recently, has increased again. This could reflect prevailing economic conditions and perceived urgency but also appeared to be linked to oil prices. When oil prices are low, carbon capture seems too expensive, so sequestration efforts and storage tend to be limited. The analysis retrieved a small number (10%) of patents related to carbon capture, indicating low commercial interest in this technology; recently, however, the numbers have shown an encouraging steep growth.
The various approaches to capture carbon fall into four categories: material science, biological, chemical, and geological.
Material science approaches
Material science approaches, including systems for carbon capture from flue gases, are summarized in Figure 3 and Table 1. Among these, post-combustion capture is the most widely used being suitable for retrofitting to flues on existing power plants but employs much energy and is therefore expensive to run. An emerging method, direct air capture, in which CO2 is captured directly from the air, could have a wide application, but this process is made more difficult by the low concentration of atmospheric CO2 and has a high cost.
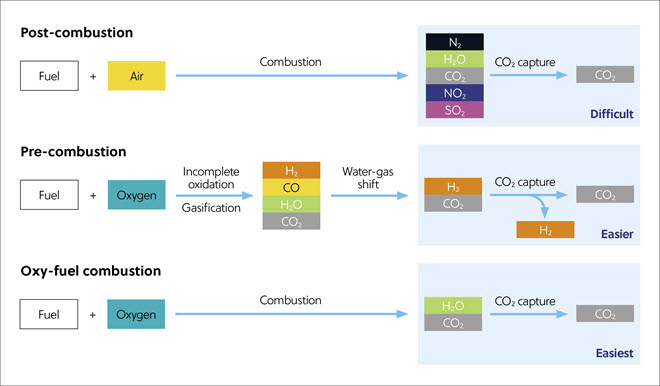
Table 1. Material science methods: comparison of CO2 capture processes
| Processes | Advantages | Disadvantages | Retrofitting Difficulty |
| Post-Combustion | More mature technology, least expensive | Low pressure stream with low CO2 concentration undermines separation efficiency, CO2/N2 separation difficult | Low |
| Pre-Combustion | High-pressure stream with high CO2 concentration, CO2/H2 separation easier | Only works for gasification or reforming plants; no industrial application yet, pure oxygen expensive | Moderate |
| Oxy-Fuel | Facile CO2/H2O separation | Pure oxygen production very costly | High |
| Chemical Looping | Facile CO2/H2O separation | Technology in early stage; more complicated process and equipment | High |
The key methods of carbon capture from flue gases are summarized in Table 2. These encompass chemical absorption with an alkaline solution and physical absorption using non-corrosive solvents such as methanol or Selexol. Additional approaches include adsorption into porous solid adsorbents, which is well studied, and membrane filtration, which is an emerging technology but is not yet widely used due to low CO2 separation efficiency.
Table 2. Material science methods: comparison of CO2 capture methods
| Method | Most Suitable Process | Advantages | Disadvantages | Maturity |
| Absorption | Post-combustion | More mature technology, lower cost, simple operation | Corrosive solvent used, high solvent loss, high energy required for solvent regeneration | Moderate |
| Adsorption | Pre-combustion | Continuous operation, environmentally friendly | Low CO2 selectivity, difficult to manage solid/gas contact to maximize adsorption capacity, too many potential candidates, actual performance of adsorbents difficult to predict | Low |
| Membranes | Post- and Pre-combustion | Simple and flexible system, environmentally friendly, no regeneration needed | Low CO2 permeability, energy intensive, membrane material easily compromised | Very Low |
Biological approaches
Biological approaches to carbon capture largely take advantage of photosynthesis which accounts for the largest influx of CO2 on Earth. Various plant materials such as wood or algae are converted to biofuels (biomass) for combustion, creating carbon-neutral and sustainable processes. Enzyme-based technologies have potential as alternatives to biosystems. A key example is 1,5-bisphosphate carboxylase/oxygenase (RubisCO) – a highly abundant and researched enzyme. Its CO2 capture, however, is naturally slow, but ongoing work aims to increase RubisCO activity to create industrially viable processes.
Chemical approaches
There are also multiple chemical methods of carbon capture, such as catalytic processes involving reduction with hydrogen, which have been widely deployed on multi-ton scales. Other well-used methods include electrochemical processes in which protons and a catalyst are used to reduce CO2. Photochemical, photothermal, and photoelectrochemical processes using clean energy are an interesting prospect, but as of yet, they are limited by efficient transfer of light energy to a substrate. Plasma-based processes also have potential but need high energy and require further development for use in carbon capture.
Geological approaches
Geological methods of carbon capture are a key solution to the long-term storage of CO2 away from the atmosphere. Captured CO2 can be compressed, transported, and injected into deep porous geologic formations or saline aquifers. This process has the capacity to store gigatons of CO2, but the selection of suitable sites is critical.
CAS literature analysis on carbon capture
The CAS literature analysis revealed a low publication rate on CO2 capture prior to 2007, but then rose to a peak during the early 2010s and then stabilized (Figure 4). There were fewer publications on pre-combustion and oxy-fuel combustion, most likely due to the economic difficulty in adapting current facilities, but these have increased more recently. Patent filing appeared to increase in 2012 and then stabilize, indicating continued commercial interest.
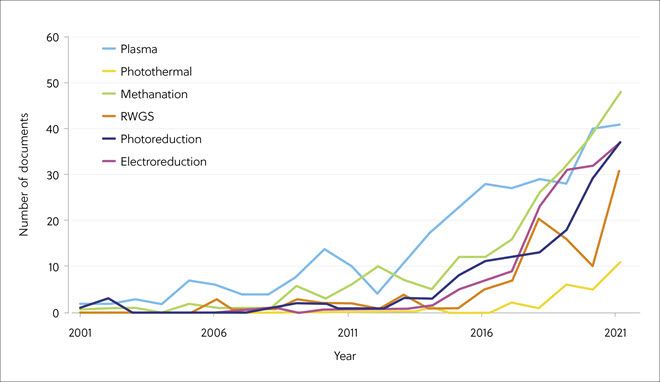
The CAS analysis also showed that publications on various chemical methods of CO2 conversion increased rapidly over the last six years compared with years before that (Figure 5). Among these, methanation, plasma-mediated processes, and reverse water-gas shift methods showed the greatest interest.
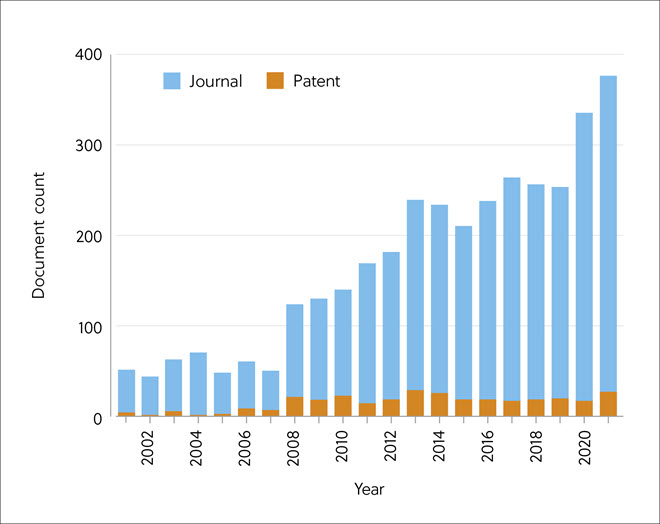
Publication numbers indicate a rapid rise in interest in biological CO2 fixation, but patent filing has been constant, reflecting a few limited technologies ready for commercialization (Figure 6). Publications on Bioenergy with Carbon Capture and Storage (BECCS), however, showed strong interest.
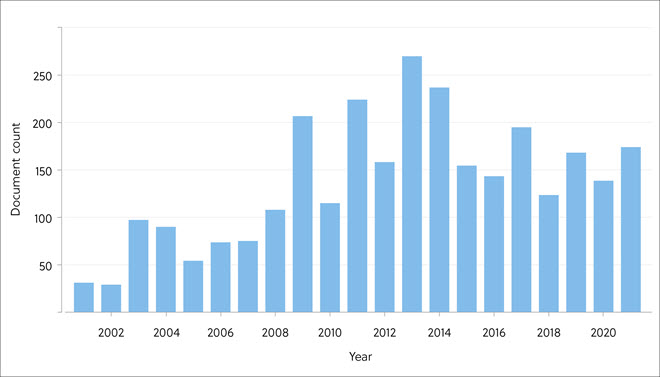
Publications on the geological storage of CO2 increased steadily and peaked in 2013 but declined thereafter (Figure 7). Search terms including ‘aquifer’, ‘saline’, ‘brine’, ‘shale’, and ‘clathrate’ found more publications than others in recent years reflecting more interest in these types of storage.
Turning the dream into reality
The CAS literature analysis of 18,500 publications indicates substantial, rapidly increasing interest in many of the different approaches to CO2 sequestration. At present, no method predominates; there are few that have been put into widespread use, but the analysis indicates considerable research effort into harnessing existing technologies and developing new ones. The numbers of patent filings were smaller than research articles but showed commercial interest in some technologies. The more recent results are likely to reflect increased public awareness of global warming and the realization that actions to counter it are imperative. The apparent correlation between research activity and economic conditions and the price of oil may diminish as urgency increases. The publication trends determined by CAS suggest that the pace of research and technology deployment is likely to continue at a rate only dreamed about in 2000 and are now driven by the realities of global warming becoming more apparent.



