Gain new perspectives for faster progress directly to your inbox.

Cows take much of the blame for food’s greenhouse gas emission problem, but plant-based foods also have a greenhouse gas footprint that no one talks about. While livestock is a big contributor, the “hidden aspects” like fertilizer production also contribute to the 13.7 billion metric tons of CO2 equivalents generated by our food system.
The fertilizer, plant supplements (nitrogen, phosphorus, potassium, etc.), and soil management practices are hidden contributors. While nitrogen, phosphorus, and potassium are crucial for agriculture, their sources, production, and supply chains contribute to greenhouse gas emissions.
See what the experts have to say on the latest scientific and market trends for phosphorus recycling and sustainable fertilizer production. Join us on November 9th at 9 a.m. EDT for a lively and informative webinar: Register
Ammonia has very large GHG emissions
While the world’s population continues to grow, not surprisingly a 2019 FAO report predicts that the demand for nitrogen for fertilizer will continue to increase. For fertilizers, traditional ammonia production methods (like the Haber-Bosch process) drive the availability of ammonia fertilizers but also generate significant carbon dioxide production. However, much of the methods to meet that demand are currently focused on traditional ammonia production practices, which would continue to increase greenhouse gas production.
Greener ammonia production is possible
There is much research underway to develop greener ammonia production. This can be done by generating its feedstock hydrogen electrochemically with sustainable power. However, chemistry offers more advanced concepts as well, for example, by either photochemical or electrochemical reduction of nitrogen. Figure 1 characterizes the direct electrochemical reduction of nitrogen. The analysis of Hochman et al found that the direct electrocatalytic approach was less expensive than the alternative with electrolysis of water and Haber-Bosch.
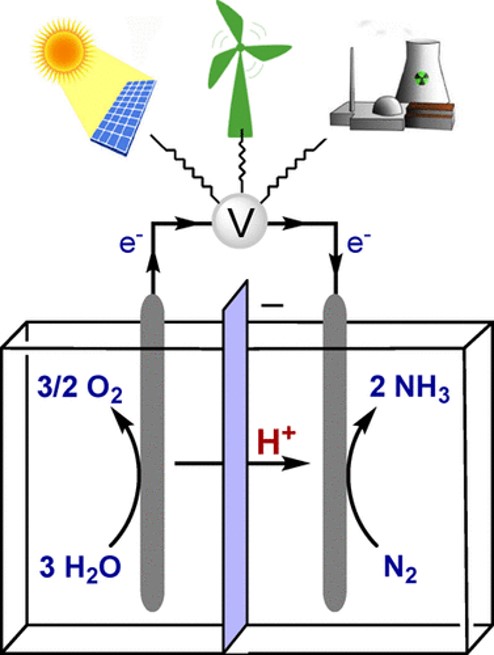
Incentivizing green ammonia production
From an economic point of view, the European Commission is planning tariffs on carbon dioxide emissions for imports of goods. The tariff plan is the EU’s solution to prevent importers from having advantages over EU-producers subject to strict climate change policies. These laws will phase in over three years commencing in January 2023 through the end of 2026.
Natural gas price instability and the current geopolitical climate are strong innovation drivers for Europe to find alternatives to natural gas-based ammonia production. Together with more affordable large-scale electrolysis to generate ammonia, this will make green ammonia competitive soon.
Reliance on phosphorus as a natural resource
The last few years have brought reports of “the phosphorus crisis.” Affordability for farmers, pollution, overuse of fertilizers, and geopolitical control of phosphorus natural resources are all recognized as part of the problem. There is also the question of the amount of phosphate rock that can be mined and how “good” is it?
This becomes a food and water security issue which will only increase with population growth. The costs of managing phosphate water pollution are high as are the toxic effects of resulting algal blooms.
Phosphorus recycling: a circular economy opportunity
Wastewater offers a prime source of secondary phosphorus: it needs to be taken out, thus also providing access for its recovery. Recycling phosphorus from wastewater, biosolids, and sewage ash starts with methods such as chemical precipitation and using microorganisms for enhanced biological phosphorus removal.
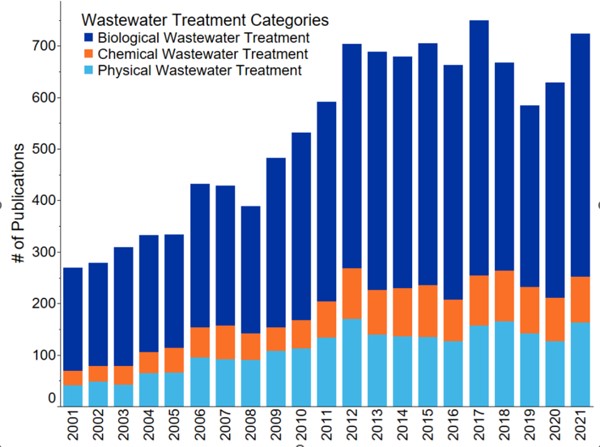
Since 2001, there has been an overall increase in published scientific literature on wastewater treatment methods associated with fertilizer nutrient recovery (Figure 1). Biological treatment processes are the most popular in the literature followed by physical methods and chemical methods. Nutrient recovery is one aspect of the overall complex process of phosphorus recycling.
Struvite precipitation is an increasingly popular method to remove phosphate from wastewater. While it improves the performance of the wastewater treatment plant – its phosphorus recovery potential is low.
However, taking phosphate out of wastewater is one side of things, but getting it back in a usable form is the next challenge, especially when targeting existing fertilizer classes. Traditional fertilizer production pathways have limited applicability to turn recovered materials into drop-in market products.
Various methods to make fertilizers out of sewage sludge or sludge ash are in an advanced stage of development. These processes often start with a phosphate-containing waste and then undergo significant chemical transformations to obtain materials that can be in the value-chain. For example, phosphorus recycling technologies can have large energy requirements (including intensive drying or concentration steps) that need to be managed.
Learn more
What do the experts say about how scientific and market trends are converging for more sustainable fertilizer production? See unique insights on greener ammonia production and phosphate recycling from Dr. Willem Schipper from Willem Schipper Consulting and Dr. Lisa Babcock-Jackson from CAS.
Register for our latest webinar “Market and Science Trends in Sustainable Fertilizers” on November 9th at 9:00 a.m. EDT.



