Gain new perspectives for faster progress directly to your inbox.
Imagine a world where the battle against cancer no longer relies solely on the blunt instruments of chemotherapy and radiation therapy, which, while effective, often come at a high cost to the patient's wellbeing. Immuno-oncology (IO) therapy has ushered in a new era by harnessing the body's innate defenses to combat cancer cells and has already proven effective in treating bladder, breast, and colorectal cancers, among others.
As with any cutting-edge field, the landscape of IO therapy is vast and multifaceted. It stretches beyond a singular approach, encompassing a rich tapestry of methodologies and strategies aimed at augmenting our immune response. From immune-boosting drugs that galvanize our body's defenses to monoclonal antibodies targeting specific cancer-related proteins, immunotherapy for cancer is a dynamic arena where innovation knows no bounds.
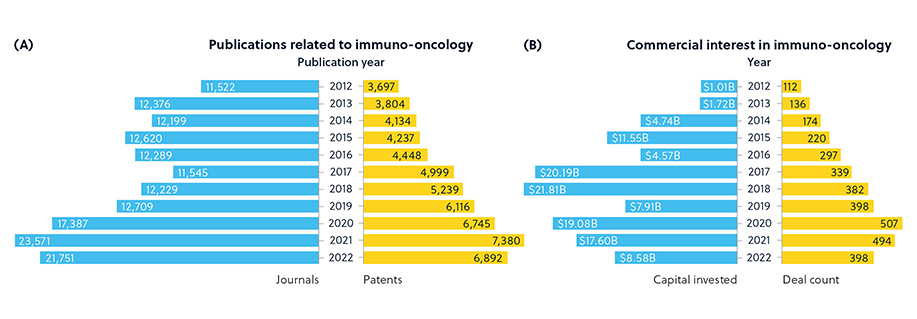
We mined the extensive CAS Content Collection™, the largest human-compiled multi-disciplinary database of documents and substances, to uncover the emerging trends and concepts in this broad and rapidly progressing field. There has been a steady increase in immunotherapy interest for cancer recently. (Figure 1).
Exploring emerging trends in cancer immunotherapy
A thorough analysis of data sourced from the CAS Content Collection in cancer immunotherapy led us to a substantial dataset comprising over 350,000 publications, encompassing both journals and patents dedicated to this field. This analytical endeavor demanded a meticulous approach, beginning with subject matter experts (SMEs) crafting a highly specific search query. This was followed by Natural Language processing, allowing the identification of phrases and rigorous manual scrutiny by our SMEs to fine-tune those phrases. Ultimately, the carefully curated set of phrases was the foundation for deriving insights into the growth patterns of publications and citations from 2003 to 2022 — a CAS TrendScape®. (Figure 2).
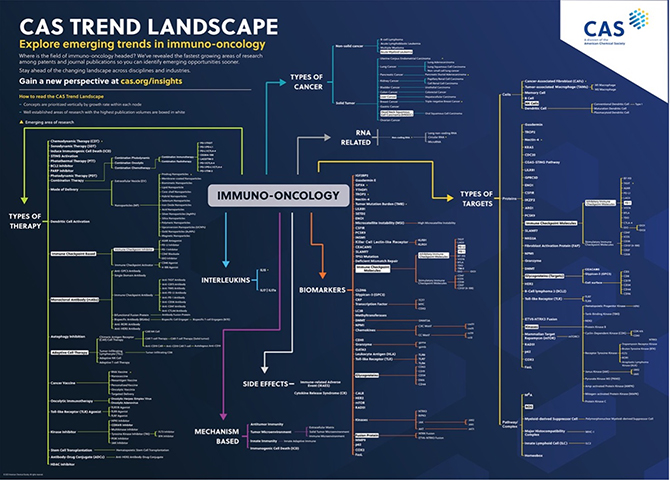
The question that naturally arises is: what has this comprehensive trend analysis unveiled, and what implications does it hold for the future of immunotherapy in oncology?
Checkpoint inhibitors: The cornerstone of cancer immunotherapy
Immune checkpoint inhibitors have sparked a transformative shift in the oncology landscape, offering newfound hope and alternatives for treating a diverse spectrum of cancers. As such, these molecules claim the lion’s share of publications.
While immune checkpoint inhibitors like programmed cell death 1 receptor (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors are widely used in clinical practice, ongoing research remains fixated on two pivotal fronts: the development of novel inhibitors and the unravelling of mechanisms behind resistance to these game-changing therapies.
However, interest in the various inhibitory immune checkpoint molecules is not growing consistently. While research into molecules such as indoleamine 2,3-dioxygenase (IDO) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) appear to be growing slowly, others such as adenosine A2a receptor (A2AR) lymphocyte-activation gene 3 (LAG-3), T cell immunoreceptor with Ig and ITIM domains (TIGIT), and B7-H3 show potential for rapid growth in the future. Thus, our trend landscape analysis provides invaluable insights into the trajectory of this pivotal immunotherapy domain for cancer, enriching our understanding of its potential.
Antibody-drug conjugates, vaccines and beyond: Exploring further cancer immunotherapy advances
Cancer immunology has evolved rapidly in the past 20 years, with shifts in emerging targets over time (Figure 3). A deeper understanding of the immune system’s role in cancer has led to the growth of other major immunotherapy treatment modalities beyond checkpoint inhibitors, including chimeric antigen receptor (CAR) T-cell therapy, antibody-drug conjugates (ADCs), and cancer vaccines.
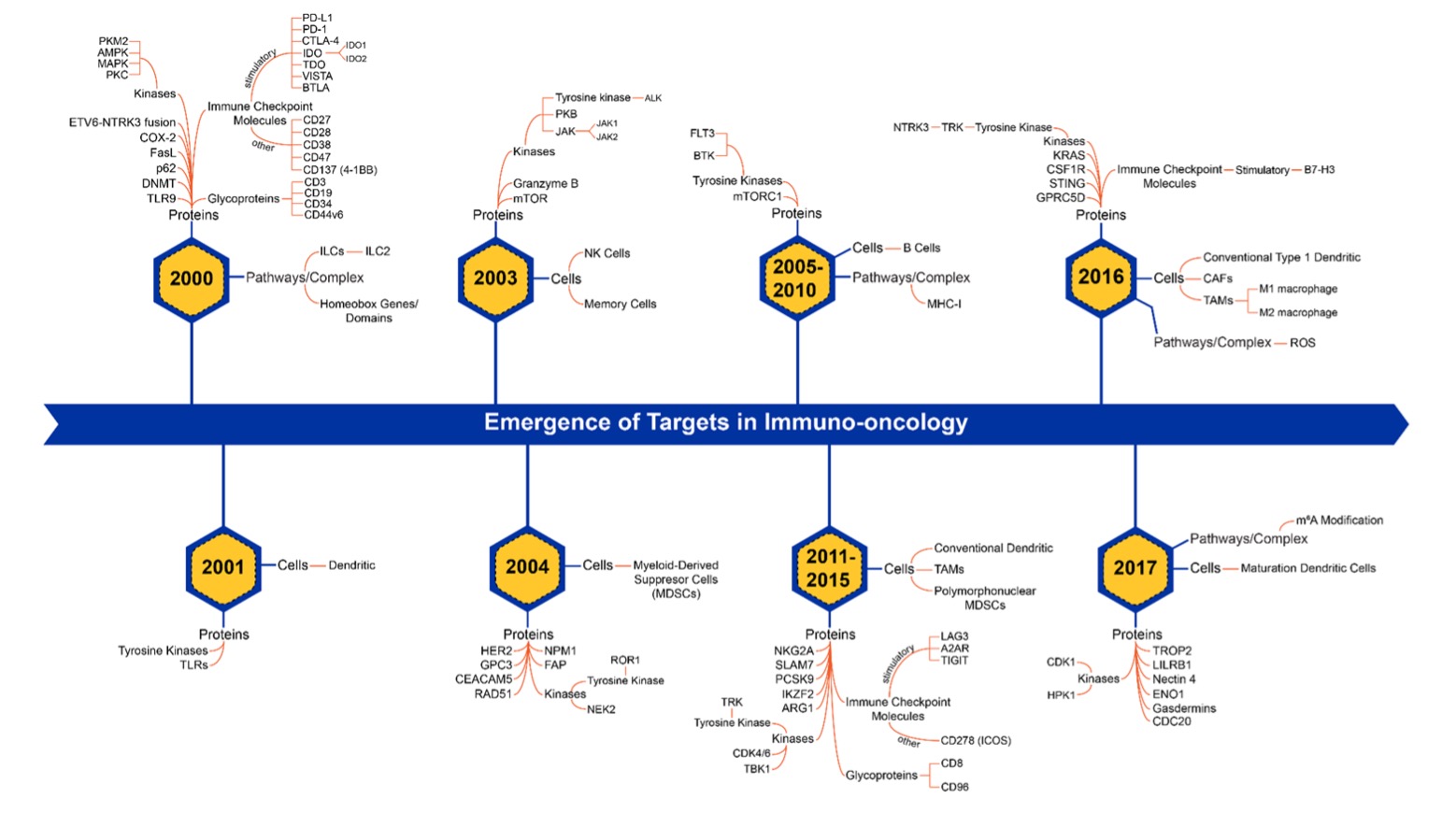
CAR-T cell therapy involves genetically modifying a patient's T cells, enabling them to recognize and attack cancer cells with specific surface antigens. Recent years have seen a swift surge in CAR T cell publications, reflecting the accelerated pace of breakthroughs, with six FDA-approved CAR-T cell therapies already in use (Table 1).
| Name | Target Antigen | Year of approval | Company | Target disease | CAS RN number |
| Kymriah (Tisagenlecleucel) | CD19 | 2017 | Novartis | B-cell acute lymphoblastic leukemia (ALL) and B-cell non-Hodgkin lymphoma (NHL) | 1823078-37-0 |
| Yescarta (Axicabtagene ciloleucel) | CD19 | 2017 | Kite Pharma/Gilead | B-cell NHL and follicular lymphoma | 2086142-87-0 |
| Tecartus (Brexucabtagene autoleucel) | CD19 | 2020 | Kite Pharma/Gilead | B-cell ALL and mantle cell lymphoma (MCL) | 2691112-12-4 |
| Breyanzi (Lisocabtagene maraleucel) | CD19 | 2021 | Juno Therapeutics, Bristol-Myers Squibb | B-cell NHL | 2099722-39-9 |
| Abecma (Idecabtagene vicleucel) | B-cell maturation antigen (BCMA) | 2021 | Celgene Corporation, Bristol-Myers Squibb | Multiple myeloma | 2306267-75-2 |
| Carvykti (Ciltacabtagene autoleucel) | BCMA | 2022 | Janssen Biotech | Multiple myeloma | 2641066-71-7 |
Table 1. FDA-approved CAR-T cell therapies.
ADCs have emerged as another formidable force in the arsenal against cancer. Here, a precision-guided approach takes center stage, as monoclonal antibodies combine with cytotoxic drugs to deliver potent cargo exclusively to cancer cells. The ADC landscape mirrors the rapid progress of CAR-T cell therapies, boasting a portfolio of over 150 unique candidates currently in clinical trials in addition to the 14 FDA-approved ADCs already in use.
Cancer vaccines work by programming the immune system to attack ‘foreign’ cancer cells. Our trend landscape analysis highlights the continued growth of ADCs and CAR-T cell therapy, along with identifying cancer vaccines as an emerging trend, with publications growing rapidly in recent years. This is a testament to the momentum behind this innovative approach, which holds the potential to revolutionize the way we use immunotherapy for cancer on a global scale.
Immunotherapy in cancer meets nanomedicine: A shifting paradigm
The effectiveness of emerging cancer immunotherapies hinges on their ability to reach tumor sites precisely. This precision is achieved through drug or cell delivery systems, a critical component in maximizing treatment impact while minimizing off-target effects. Furthermore, drug delivery systems can facilitate the simultaneous delivery of multiple therapeutic agents. This opens the door to synergistic effects when combined with other cancer treatments like chemotherapy, radiation therapy, or other IO therapies.
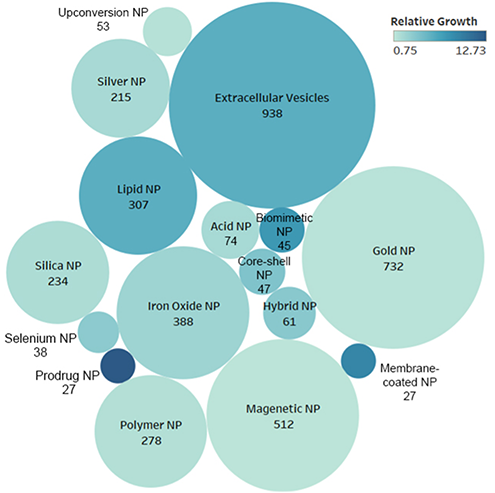
In recent years, materials science and nanotechnology advances have given rise to various nanomaterials. While lipid nanoparticles have been the workhorses in immunotherapy delivery, our trend landscape analysis indicates promising developments. Prodrug delivery systems, biomimetic designs, and cell membrane-coated nanoparticles are emerging as noteworthy nanotechnologies (Figure 4).
The future of cancer immunotherapy: Unveiling promising horizons
In the ever-evolving landscape of immuno-oncology, the journey is far from over; it's gaining momentum with each stride. With each new advance, emerging targets and biomarkers that hold the key to exciting IO therapy innovations are being continually uncovered. These findings are exciting and a testament to the untapped potential of immunotherapy in cancer.
To learn more about the ever-evolving immuno-oncology landscape, see our peer-reviewed publication.