Check the spelling in your query or search for a new term.
Site search accepts advanced operators to help refine your query. Learn more.
CAS STNext® User Meeting | The Netherlands
CAS STNext® User Meeting | The Netherlands
Share:
PEGylation is a breakthrough technique to improve drug delivery systems, but it also has a downside: it can trigger immune reactions that reduce drug efficacy and safety. Designed for R&D leaders, this idea in brief shows how we can overcome this challenge. It summarizes the latest trends and opportunities, PEG-lipid structure, LNP composition and properties, and which pharmaceutical parameters affect their immunogenicity and efficiency. Learn more and share with your network:

Gain new perspectives for faster progress directly to your inbox.
Gain new perspectives for faster progress directly to your inbox.
Related Insights
Share:
Gain new perspectives for faster progress directly to your inbox.
Share:
The power of polyethylene glycol
Polyethylene glycol (PEG) is a flexible, non-toxic, hydrophilic polymer with a broad range of applications, from personal care products to pharmaceutical formulations. PEG-lipids have been widely used in pharmaceutical lipid nanoparticle (LNP) formulations for anti-cancer medications such as doxorubicin, irinotecan, and cisplatin, as well as small-interfering RNA patisiran and the messenger RNA vaccines developed by BioNTech/Pfizer and Moderna. The modification of PEGylated pharmaceuticals is a widely implemented approach to reduce clearance by the reticuloendothelial system, extend circulation time, improve pharmacokinetics, and enhance drug efficacy.
However, studies have reported unexpected immune responses against PEGylated nanocarriers. Furthermore, hypersensitivity reactions, including anaphylaxis, have been reported in association with many PEG-containing formulations. This article explores how various structural parameters of the PEG-lipids affect the immune responses and activities of the LNPs regarding their efficiency in drug delivery.
Growing research interest in PEGylated proteins
The global PEGylated proteins market is expected to expand significantly in the next five years, being estimated to reach $2.1 billion by 2028. This growth is largely driven by the rising rate of cancer worldwide, though the technology is increasingly being adopted for other disease areas.
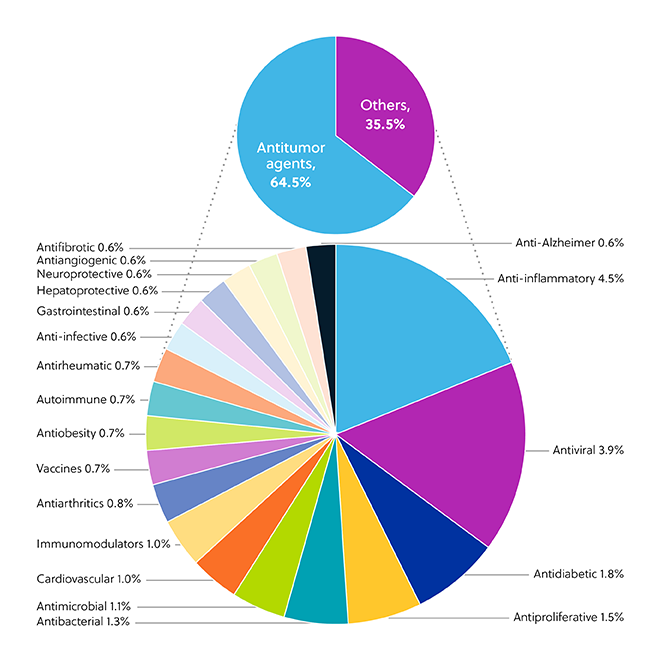
PEGylated LNP formulations are widely explored as therapeutic options against various diseases and disorders and are extensively represented in the CAS Content CollectionTM. While nearly two-thirds of LNP applications (64.5%) are in cancer, other notable targets are anti-inflammatory (4.5%) and antiviral (3.9%) medications (Figure 1).
Polyethylene glycol is considered to have low immunogenicity. Yet, there is growing evidence that it initiates immunogenic responses, especially when conjugated with other materials such as proteins and nanocarriers. Interestingly, anti-PEG antibodies can be found in the general population in individuals who likely have never received systemic PEGylated therapeutics. Furthermore, some PEG-modified compounds induce additional antibodies against polyethylene glycol, which can adversely impact drug efficacy and safety.
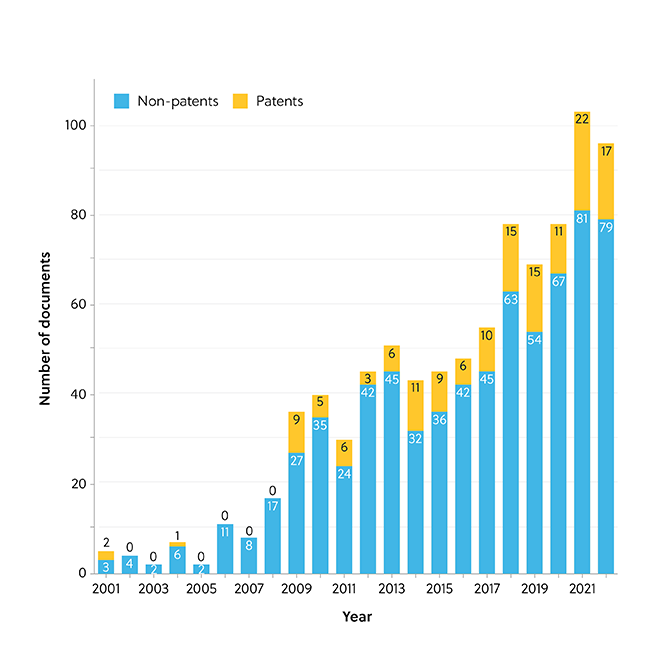
With over 50 million SARS-CoV-2 vaccination boosters administered in the U.S. to date, several questions have arisen about the immunological safety of polyethylene glycol, including PEGylated LNPs. Anaphylaxis was reported to occur in a small number of people (2.5–4.7 per million as of April 2022) shortly after the administration of the Pfizer-BioNTech (Cominarty®) and Moderna (Spikevax®) COVID-19 vaccines. Data from the CAS Content Collection shows yearly growth in the number of documents related to PEG-lipids and their immunologically induced adverse effects up to and including 2021 (Figure 2).
Understanding the immunogenicity of PEGylation
Accelerated blood clearance (dubbed ‘the ABC phenomenon’) is an unexpected immunogenic response observed in PEG-conjugated substances that causes the rapid clearance of PEGylated nanocarriers. The ABC phenomenon has been extensively observed upon repeated administration, resulting in diminished efficacy of PEG-conjugated substances and nanocarriers.
Another unanticipated immune response is a hypersensitivity reaction referred to as CARPA, which significantly reduces the safety of PEGylated nanocarriers and is associated with reduced efficacy of PEGylated therapeutics in clinical trials. The CARPA phenomenon has been classified as a non-IgE-mediated pseudoallergy caused by the activation of the complement system.
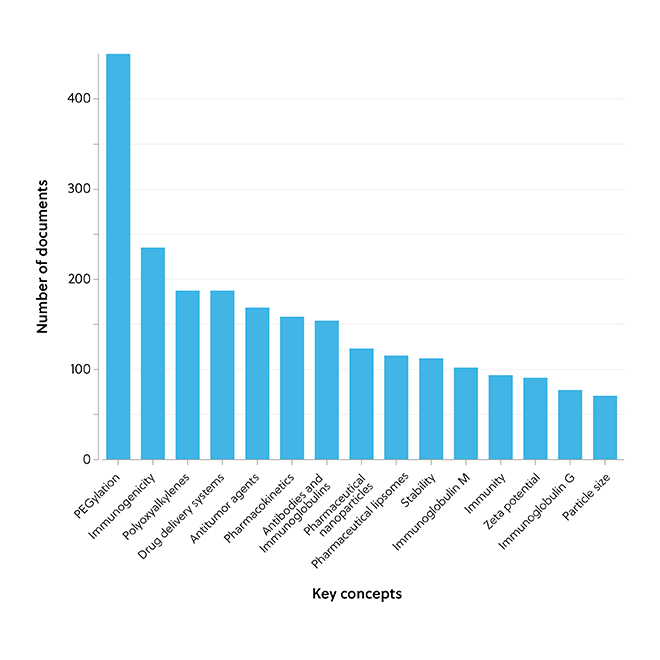
The association between PEGylation and immunologically induced adverse effects of ABC and CARPA is supported by data from the CAS Content Collection, which highlights that PEGylation is a key concept related to these adverse effects (Figure 3).
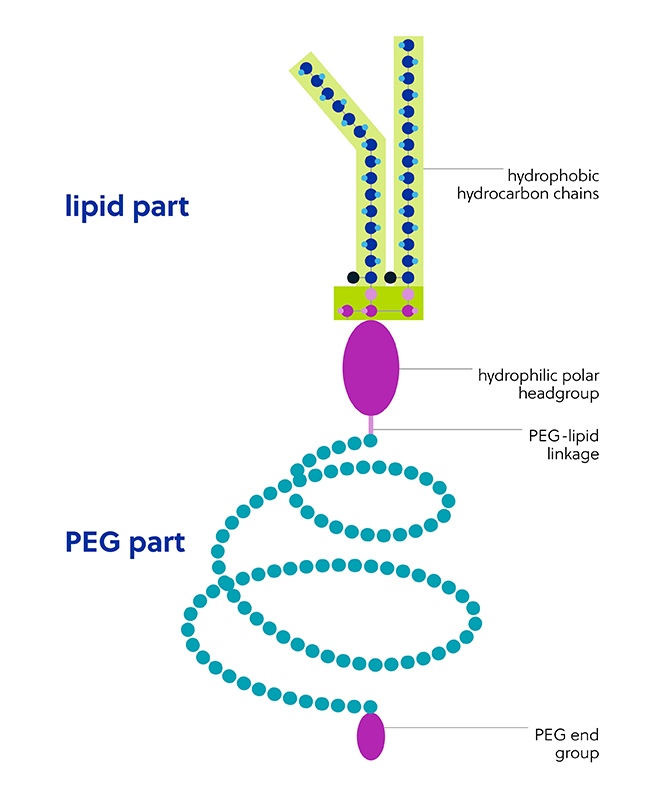
The polyethylene glycol part of the PEG-lipid structure is extremely hydrophilic, flexible, and mobile. Components of the PEG-lipid chemical structure (Figure 4) contribute to the improved stability of LNPs but may also impact their safety and efficacy:
-
PEG length is a key structural factor that impacts immunological safety. The effect appears biphasic, with both long- and short-chain PEG conjugates shown to be more likely to induce the ABC phenomenon.
-
Like PEG length, PEG density (i.e., the percentage of PEG in LNPs) also exhibits a biphasic effect. However, it is both lower and higher densities of PEG that exhibit a reduced ABC phenomenon.
-
Differences in PEG architecture can have an impact, with branched PEG-lipid conjugates conferring higher stealth properties to LNPs than linear PEGs.
-
Functional terminal groups appended to PEG particle chains are an additional factor that impacts their immunogenicity and rate of clearance.
-
Parameters such as size and surface charge can also impact immunogenicity. For example, PEGylated carriers comprising negatively charged phospholipids can stimulate the immune system via complement activation to a greater extent than uncharged vesicles.
-
Like the PEG part, the lipid hydrophobic chain structure and length can also determine the extent of immunogenic effects, but also efficacy.
-
The specific lipid anchoring group employed (e.g., cholesterol as the anchoring group) yields PEGylated LNPs with longer permeance in the circulation and higher systemic bioavailability.
-
Lipid linkage is an important parameter in lipid design and performance, where the substitution of an ester linkage for a carbamate linkage allowed for the formation of unstable vesicles.
Enhancing the safety and efficiency of polyethylene glycol in medicines
While PEGylation has become a gold standard in pharmaceutical nanocarrier modification for developing successful drug delivery systems, immunosafety is a key issue in current research and development of nanomedicines such as LNPs. In fact, there are currently more than 200 clinical trials registered on ClinicalTrials.gov to examine PEGylated lipid safety, mainly PEGylated liposomal doxorubicin in various solid tumors and the mRNA SARS-CoV-2 vaccines, Comirnaty®, and Spikevax®.
Understanding the factors that impact anti-PEG antibody production is crucial for both researchers and clinicians to develop novel drug vehicles or to adjust the route of administration and injection schedule to ensure the highest treatment efficacy.
An array of alternative polymers, such as poly(oxazoline), polyvinyl alcohol, and poly(glycerol), have been investigated to overcome immunogenic issues with polyethylene glycol. Despite proven benefits, no agent has yet been proven to be superior to PEG in terms of augmenting the pharmacokinetic performance of LNPs, and they carry their own hypersensitivity risks. Other alternative polymers that mimic the stealth properties of polyethylene glycol, including zwitterionic and hydrophilic polymers, are currently in development.
Though recent research has helped elucidate many of the factors that contribute to immunogenicity, the immune toxicology of nanomedicines is a largely unexplored research area at a broad intersection of nanotechnology, immunology, and pharmacology. Improved knowledge in this area can enable us to develop optimal pharmaceutical formulations that diminish undesirable immune reactions and enhance the safety and efficiency of PEGylated medicines.
Read our Executive Summary or our in-depth, peer reviewed journal publication in Bioconjugate Chemistry.
Gain new perspectives for faster progress directly to your inbox.
Gain new perspectives for faster progress directly to your inbox.
Related Insights
Share:
Gain new perspectives for faster progress directly to your inbox.
Five ways to achieve sustainable medical packaging
Share:

From individuals to large corporations, many today are looking for ways to switch to more sustainable products and processes. Eco-conscious packaging, which minimizes waste and prioritizes the use of recyclable and biodegradable materials, has become the new normal in much of our lives. However, when it comes to creating sustainable medical packaging, the industry faces a host of unique challenges.
Plastics manufacturers play a key role in creating sustainability by developing packaging options for medical device manufacturers and practitioners to implement. When tasked with designing innovative packaging solutions that can carefully account for the intricate needs of medical devices, it is easy to ask—is it possible to achieve sustainability in medical packaging? Our answer: Absolutely.
1. Reduce, reuse, recycle

Despite having industry-specific challenges to sustainability, the medical sector has several areas where classic sustainable choices can be made. In such a high-tech business, more generalized sustainability options can be overlooked, but they still contribute to a well-rounded approach.
These kinds of policies can include:
- Reducing any unnecessary packaging, including creating more strategic branding choices that minimize the quantity of packaging needed.
- Designing product lines that include reusable containers and ‘refill’ versions of the product to facilitate this reuse.
- Switching to recyclable or biodegradable material options for packaging to reduce the amount of landfill waste produced.
The plastics industry has a particular stake in these policies. Traditional plastics are not biodegradable and their use causes pollution through both landfill contribution and microplastics contamination. To avoid these environmental consequences, there is a push toward a reduction in packaging quantity and the use of recyclable or biodegradable replacements for plastics. Because of this, the development of novel polymers and recycling methods are key to the future of plastic packaging.
Experimentation in recent years has produced some interesting advances in plastic technology which could be utilized for sustainable medical packaging, such as:
- The engineering of a plastic-degrading depolymerase enzyme that can be used to reduce waste and increase the circularity of poly(ethylene terephthalate) (PET).
- A novel bioplastic material that could be used as a packaging material.
- Increased accessibility and efficiency of depolymerization with nano-dispersion of enzymes.
The success of these technological advances relies heavily on their cost-effectiveness and scalability. The use of depolymerase enzymes for PET degradation has hit this barrier with enzymes such as leaf-branch compost cutinase, which denatures at high temperatures, limiting its scalability. Luckily, the development of new technologies like PET degrading super-enzymes, such as PET hydrolase (PETase), offers the promise of financially and environmentally sustainable scalability.
2. Develop sustainable sterile packaging

One of the key challenges to sustainability in medical packaging is its reliance on sterile packaging. Ensuring the sterility of contents is paramount to patient safety and cannot be compromised. Packaging needs to meet ISO standards of providing a sterile barrier. Because of this, plastics, which can be easily shaped and provide a fully sealed, waterproof, and non-reactive sterile environment, are an ideal material for sterile packaging.
Unfortunately, much of this packaging is single-use and not recycled, creating enormous amounts of waste. One 2022 study in a German hospital found that plastic packaging accounted for 16g of waste per patient per day. This massive consumption of plastic for packaging highlights how important it is to innovate in this area.
However, the weight of creating sustainable medical packaging for sterile purposes does not lie only with the plastics industry. Manufacturers have pushed toward using recyclable plastics, but recycling efforts require the infrastructure to facilitate the use of these sustainable waste streams. For example, any packaging opened in a surgical theater after the operation has begun is required to go into the biohazard stream of waste. To recycle, the packaging must be opened into a separate waste bin, which is removed before the patient is in the theater. While this is possible in many cases, it requires medical practitioners to push for this kind of change within operating rooms.
With the recyclability of sterile packaging unlikely or impossible in some cases, plastics manufacturers can go further and reduce or replace plastic content where possible. This can be achieved by minimizing plastic packaging to only what is necessary or by developing bioplastic alternative materials including the following:
| Starch | Plant starches with added plasticizers are used to create either flexible or rigid pharmaceutical packaging with a controllable lifespan such as trays. |
| Cellulose | Plant-based cellulose is used to create multiple bioplastics including cellulose acetate used in laboratories and the pharmaceutical industry. |
| Chitin/Chitosan | Sourced from invertebrates or yeasts to create antimicrobial packaging, chitin can be deacetylated into its derivative, chitosan. |
| Xylan | Derived from plant cell walls and algae to create pharmaceutical packaging. |
| Protein | Various plant and animal sources with modified side chains to create synthetic polymers used in packaging. |
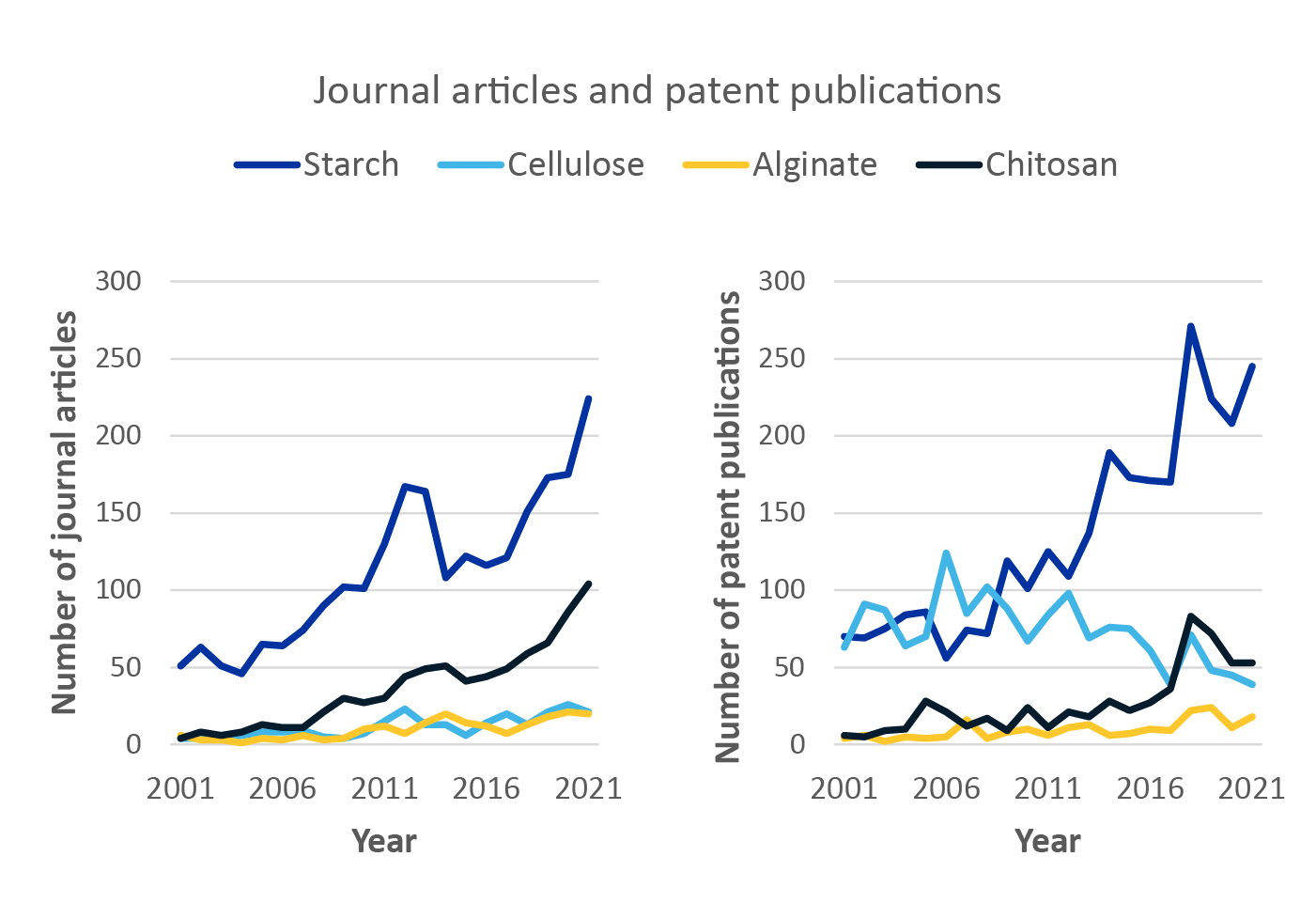
Trend analysis of these bioplastic alternatives shows that starch-based bioplastics have emerged as the frontrunner in journal and patent publications over the last 20 years (see figure below). Chitosan has also become increasingly popular, displaying an upward trend in the number of journal articles and patent publications. The development of bioplastics that are not petroleum-based and do not produce the same harmful waste product when burned in biohazard waste streams will play a key role in the future of sterile medical packaging.
3. Replace protective packing materials

While the overall aim of sustainable medical packaging is to reduce its quantity as much as possible, the transportation of delicate equipment usually requires large amounts of protective packing. Delicate or sensitive medical equipment is often expensive and occasionally irreplaceable, so ensuring it is packed safely for transportation is non-negotiable.
Since the quantity of packaging cannot be reduced, it is necessary here to make strategic sustainable material choices. The widespread use of polystyrene for this purpose produces landfill waste and microplastic contamination, but luckily there are sustainable packing options already in use that could be adopted by medical device manufacturers. Packing can largely be made of recycled or biodegradable materials with the use of paper bubble wrap, shredded cardboard, and biodegradable foam peanuts.
Packing material works primarily on the basis of volume and is unlikely to become contaminated by biohazards, so there is also room here for circularity within plastics manufacturing. If companies can find ways to reuse packing and recycle their high volumes of waste into new packing materials, they can create a much more sustainable life cycle for plastic-based packing.
This would require initiatives to reuse packing materials across the medical sector as well as the development of recycling pipelines within plastics manufacturing companies to create new packing and recycle the old. Packaging suppliers could develop closed-loop recycling of waste packaging from their own products into packing materials, increasing sustainability and creating new revenue streams.
4. Innovate temperature-controlled transportation

Packaging in the medical industry is not only for protection. Many items need to be transported in packaging that maintains a particular temperature. This is a major barrier to sustainable medical packaging due to the widespread use of unsustainable refrigeration methods and insulation materials.
Just as with packing materials, the sustainability of insulated packaging can be improved by the use of recyclable or biodegradable materials. Sustainability can be further increased by developing more effective insulating materials, which reduces the overall amount of insulation needed. Thus, the development of novel polymers to create both more effective and more environmentally friendly insulating material options could be an exciting opportunity for plastics manufacturers.
Sustainable insulating materials using either plastic alternatives, such as cardboard or plastics with a high recycling value, are starting to become available on the market, but there is still room for high-efficacy, sustainable, insulating materials tailored to meet the specific needs of the medical sector.
Along with insulation, cold chain distribution requires the use of temperature-controlled shipping services and freezers. Improved efficacy of insulation in packaging could increase the amount of time they can be stored outside of these, reducing the reliance on high-energy cost refrigeration.
5. Ensure life cycle sustainability

Materials use is only part of the story in achieving sustainable medical packaging. We must secure sustainability for the entirety of the material’s life cycle. During the development of recyclable or biodegradable polymers, plastics manufacturers need to take into account factors including:
- Raw material scarcity.
- Transportation.
- Manufacturing energy consumption.
- Recycling accessibility and byproducts.
For bioplastics, plant-based alternatives are popularly touted as fully sustainable. However, the use of starch, cellulose, xylan, chitin, and protein-based polymers needs to be underpinned by sustainable and renewable farming practices to supply these materials.
PLA (polylactic acids) plastics are created from plant starch and were originally advertised as compostable and carbon-neutral alternatives to plastic. Unfortunately, criticisms quickly grew surrounding the realistic sustainability of PLA, since it requires separating from other waste and transportation to special composting facilities. While PLA is technically decomposed in these facilities, the process is much more akin to recycling, making its life cycle similar to traditional plastics.
PLA’s story highlights the importance of the design process of new sustainable materials taking the entire life cycle into account. Ensuring the real-term sustainability of new materials will help to secure their value and popularity in the long term.
Is sustainable medical packaging achievable?

While plastics manufacturers have to overcome challenges with requirements for sterile, delicate, or temperature-sensitive medical products, there is a huge scope and opportunity for innovation. The development of new bioplastics and novel plastic recycling technologies opens the door to the sustainable future of medical packaging. When combined with initiatives from medical device manufacturers and practitioners designed to keep recycling and sustainability at the heart of packaging life cycles, these technologies could prove to be the path to a new, more sustainable future in the medical sector.
Gain new perspectives for faster progress directly to your inbox.
Gain new perspectives for faster progress directly to your inbox.
Related Insights
Share:
Gain new perspectives for faster progress directly to your inbox.
Share:
Lipid nanoparticles have redefined what’s possible in drug delivery, from recent acclaim in COVID-19 vaccines to a storied history of novel drug delivery mechanisms. However, it may come with a price: immune reactions that can compromise the safety and efficacy of PEGylated lipid nanoparticles. With a clinical pipeline full of exciting new RNA therapeutics, how can we overcome this? Learn more about the emerging trends, opportunities, and challenges in our latest peer-reviewed publication in Bioconjugate Chemistry.
Gain new perspectives for faster progress directly to your inbox.
Gain new perspectives for faster progress directly to your inbox.
Related Insights
Share:
Gain new perspectives for faster progress directly to your inbox.