Therapeutic fusion proteins have emerged as a promising class of biotechnology products, thanks to rapid advances in recombinant DNA and protein engineering technology. Since etanercept (Enbrel)—the first fusion protein drug—was approved by the FDA in 1998, sales of fusion protein drugs have increased to such an extent that they are now among the top four most lucrative classes of biopharmaceuticals. Indeed, total global sales of fusion proteins have been growing at a rate of around 6 percent annually, with the market predicted to reach an estimated 24 billion USD by 2025.
According to Yingzhu Li, Ph.D., Information Scientist and a contributing author on this blog post, organizations seeking innovative initiatives to accelerate the delivery of drugs to patients must stay abreast of the rapid developments in this area. CAS has collaborated with the National Science Library, Chinese Academy of Sciences (NSL) to produce a whitepaper on therapeutic fusion proteins that's designed to give you the complete picture of the therapeutic fusion protein landscape and help you to identify the most promising opportunities. Here we've provided some key highlights from this useful resource, and how these insights could help your business gain the competitive advantage.
How is global therapeutic fusion protein R&D evolving?
The last 30 years have seen considerable growth in fusion protein R&D. By the end of 2017, a whopping 50,164 patent applications and 12,198 papers had been published on therapeutic fusion protein topics, with particularly striking growth in the number of patents filed over the past few years. The U.S. leads the field in the number of journal articles and patents published, while China, Germany, Japan, the UK, and South Korea are also among the top contributors.
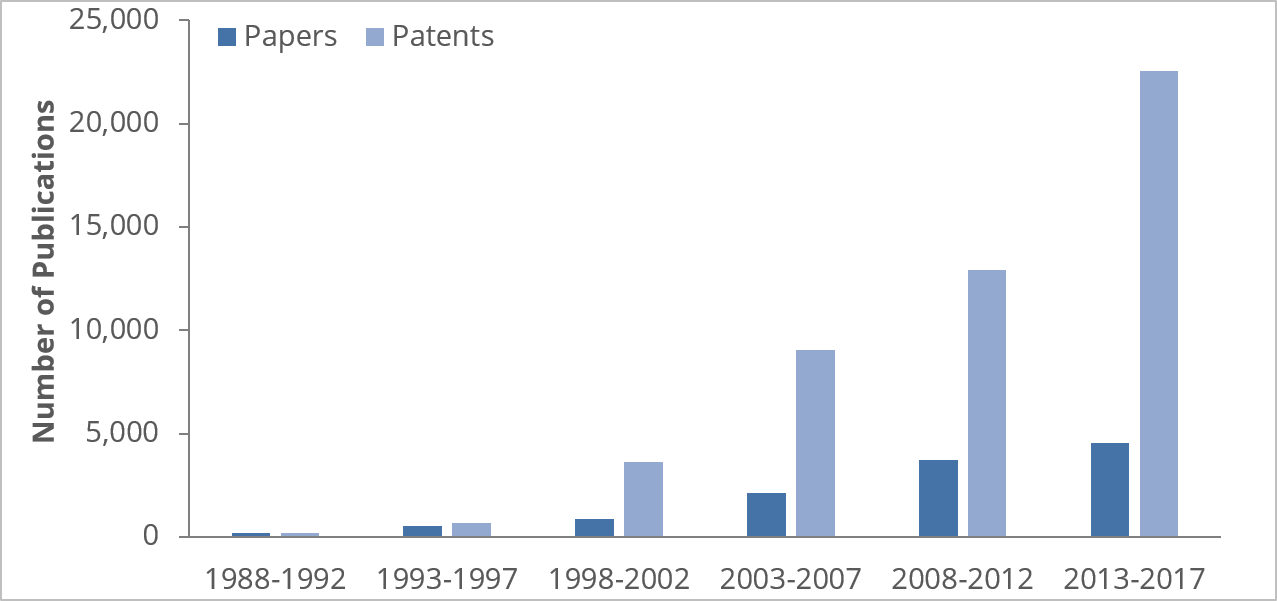
What does this mean for innovators? Well, insight into the number of patents published could allow you to determine where the market is expected to take off, where innovation shows signs of slowing, and potentially where there's greater opportunity for innovation. Looking at research published by country could also help identify potential geographical hotspots, helping you plan how to enter the market, grow your ideas through collaboration, or even be in the right place to attract top talent.
What are the major fusion protein techniques and how are they being used?
One of the biggest reasons why therapeutic fusion proteins are currently receiving so much R&D interest is their ability to extend protein and peptide drug lifetimes. By fusing biologically active proteins or peptides with a long half-life protein, the resulting fusion protein can have a significantly longer lifetime than that of the original drug. A wide range of fusion techniques have been developed, including those based on:
- Fusion of a protein/peptide drug with an antibody Fc fragment (i.e., an immunoglobulin fragment crystallizable region)
- Fusion with a recombinant serum albumin
- Fusion with transferrin
- Fusion with carboxy-terminal peptide
- Fusion with XTEN (recombinant polypeptide with features of both proteins and polymers and a larger size than globular proteins of similar molecular weight)
- Fusion with ELP (elastin-like peptide)
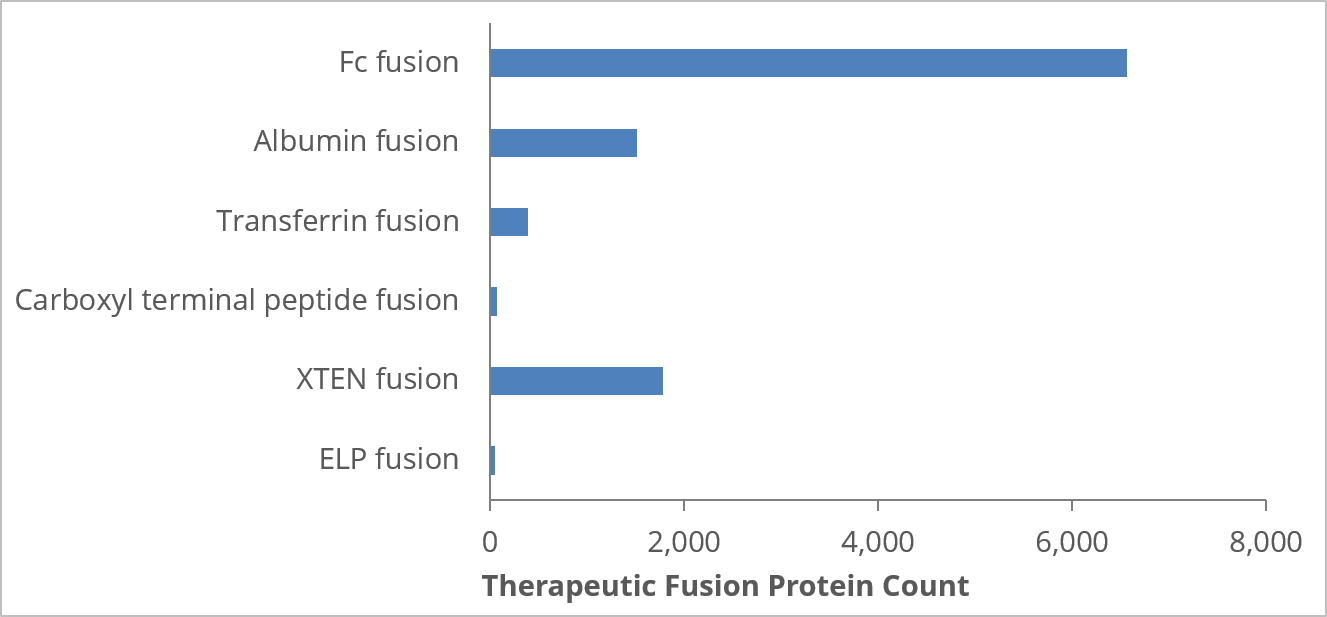
Our analysis based on CAS-registered fusion proteins shows that Fc-fusion is by far the most widely used approach. Interestingly, the more recent XTEN technique, which was developed just a few years ago, seems to be catching up quickly. Whether you're a small biotech or a more established pharmaceutical company, knowledge of R&D trends and the techniques that are delivering the greatest impact could help you identify opportunities to get ahead of the competition.
What's the next big thing in fusion protein drugs?
Part of the appeal of fusion proteins is that they can be designed to interact with multiple targets, and thus act simultaneously on two or more different pathways involved in disease development. The ability to construct fusion proteins with bi- or multi-functional specificity is particularly appealing and is a major focus of ongoing therapeutic fusion protein R&D.
Analysis of the bi-specific targets for the antibody components in therapeutic fusion proteins reveals that the most widely studied bi-specific targets are CD19 and CD3, interleukin 1α and interleukin 1β, as well as epithelial growth factor receptors and CD3. Our therapeutic fusion protein whitepaper identifies the most commonly studied fusion proteins in terms of their half-life extending components, activity components, targets (or bi-specific targets) and mechanism of action. It also provides an overview of innovative strategies that have been used in the development of fusion proteins. Taken together, this knowledge could help your business more fully understand the therapeutic protein landscape, and identify potential opportunities for growth (as well as the targets that are already receiving the most attention from competitors).
How might fusion proteins be used in future?
Currently, the major indications for therapeutic fusion drugs include inflammation, immune diseases and musculoskeletal conditions. However, fusion proteins are not only being used as drugs themselves, they've also helped ignite excitement around gene and cell therapies. Take chimeric antigen receptor T cell-based therapies, for example, in which a cancer patient's T cells are re-engineered to contain a chimeric antigen receptor, and infused back to the patient to identify and kill cancer cells.
Given the early clinical success of this strategy, it is highly likely that more innovations that utilize fusion proteins will emerge in the coming years. Using the right scientific search tools to identify relationships between therapeutic fusion proteins and disease indications could help you recognize areas where these drugs offer the greatest opportunities.
Don't let a lack of R&D insight hamper your ability to maximize the full therapeutic potential of fusion protein drugs. Download our whitepaper to see the bigger picture in this rapidly expanding market.