Updated: December 22, 2020
Recent promising news on the efficacy and safety of COVID-19 vaccines provides a light at the end of the tunnel of the pandemic that has upended normal life around the globe throughout 2020. Two mRNA vaccines, one from Pfizer/BioNTech and the other from Moderna, just received Emergency Use Authorization (EUA) in the U.S. and have also been authorized in the U.K and Canada, and there are many additional candidates following closely behind. However, with multiple vaccines expected to become available to the public in the coming months, for many people this hope comes with a multitude of questions. Here, I will answer some of the most common scientific questions about the current COVID vaccine candidates that I am being asked and point you to key resources for additional information. I encourage everyone to share accurate, scientifically vetted information sources such as these with others to help ensure they can make well-informed decisions about vaccination.
What major types of vaccines are in development for COVID-19?
There are over 200 COVID-19 vaccine candidates in development around the world based on multiple different vaccination approaches. All vaccines work by giving the human body a weaker or non-disease-causing pathogen (or antigen) similar in structure to the disease causing pathogen, so that the human immune system can be trained to recognize that specific pathogen and respond to it effectively when encountering the actual pathogen. However, vaccine platforms differ in what antigen is used and how they introduce that antigen to initiate our bodies’ immune response.
mRNA vaccines are relatively new in the vaccine research field. However, this technology has already shown great promise in vaccines against Zika virus and cytomegalovirus. The Pfizer/BioNTech vaccine (BNT162b2) and Moderna vaccine (mRNA-1273) candidate, which are expected to be the first made widely available, both use this vaccine approach, and there are more than 20 additional mRNA COVID-19 candidate currently in various stages of development.
Instead of directly delivering an immune-triggering antigen into the human body, mRNA vaccines deliver a piece of mRNA with the genetic code that our cells can use to make the antigen. mRNA is a fragile biomolecule that is easily degraded at increased temperatures or by enzymes in the human body. Therefore, these vaccines must be stored at very low temperatures. The mRNA in these vaccine formulations is also packaged in lipid nanoparticles that preserve their stability and help facilitate delivery of the mRNA to human cells.
Despite limited experience with mRNA vaccines, they are generally considered safer than traditional vaccines that use viral material because they minimize the risk of introducing pathogens or causing genomic mutations. The manufacturing of mRNA vaccines is also more rapid, less expensive and more scalable than other vaccine types, which is especially important given the urgency of the current COVID-19 situation.
For more detailed information about mRNA vaccines, read this recent blog and watch the video below that shows how these vaccines work at the cellular level.
Non-replicating viral vector vaccines use a non-disease causing virus as a vector to carry the genetic instructions for expressing the antigen protein of the disease-causing virus, yet this carrier virus has been genetically modified so that it cannot replicate in our bodies. One good example of this vaccination technology in use is the Ebola virus vaccine, which was recently licensed for emergency use in the European Union. There are ~30 COVID-19 candidate vaccines being developed based on this vaccine platform, including the leading candidates from AstraZeneca/Oxford (AZD1222) and Janssen (Ad26COVS1). This vaccine approach offers several advantages including high production capacity, low cost and less stringent temperature requirements during storage and transportation.
Protein subunit vaccines differ from both the mRNA and non-replicating viral vaccines because they introduce the antigen proteins that trigger an immune response directly into the body, instead of introducing genetic information that our cells use to make these antigens in vivo. The FluBlok vaccine for influenza is an example of a currently licensed vaccine that uses this approach. Currently, there are ~70 COVID-19 candidate vaccines being developed based on the protein subunit vaccine technology, with more than 10 in clinical trials.
Inactivated virus vaccines are produced by growing the pathogenic virus via cell culture that is then inactivated and introduced into the body. Most common influenza vaccines use this approach. Currently, there are about 20 COVID-19 candidate vaccines being developed based on an inactivated virus vaccine approach. Because the whole virus is introduced to the body, this approach applies to wide range of antigenic proteins and often mimics the true infection in terms of immune response. However, production capacity is limited because it requires a facility with a high biosafety level to support handling of the live virus.
Further comparison of each of the vaccine types and more information on how they work can be found in this article.
How was a COVID-19 vaccine developed so fast?
Traditionally vaccines have often taken 15 years or more to get from the lab to the market, including design, development, pre-clinical testing, clinical trials, and regulatory review. Thus, the fact that we have multiple highly effective vaccines for COVID-19 approved or near approval in less than a year is remarkable. This record-breaking speed does not reflect a lack of diligence in development or testing. No steps were skipped or regulatory standards lowered in the development of these vaccines. Instead, the development process was able to be accelerated in the following ways: (1) Researchers have collaborated and shared information to an unprecedented degree, starting with Chinese scientists releasing the genomic sequence of the SARS-CoV-2 virus to the global research community in early January. (2) Although the mRNA vaccine is considered a new technology, the foundational idea actually emerged in the 1990s, and research on mRNA vaccine technology that has been ongoing since 2003 with the SARS outbreak was heavily leveraged for COVID-19. (3) COVID-19 vaccine development has been actively supported from a financial and regulatory perspective by global governments, with efforts such as the Operation Warp Speed in the U.S. prioritizing these vaccines to move them through the regulatory process as quickly as possible and pre-planning for distribution. (4) The widespread nature of the pandemic and high public interest provided an adequate pool of participants to support multiple simultaneous late-stage clinical trials.
What COVID-19 vaccines will be available soon, and which is the best?
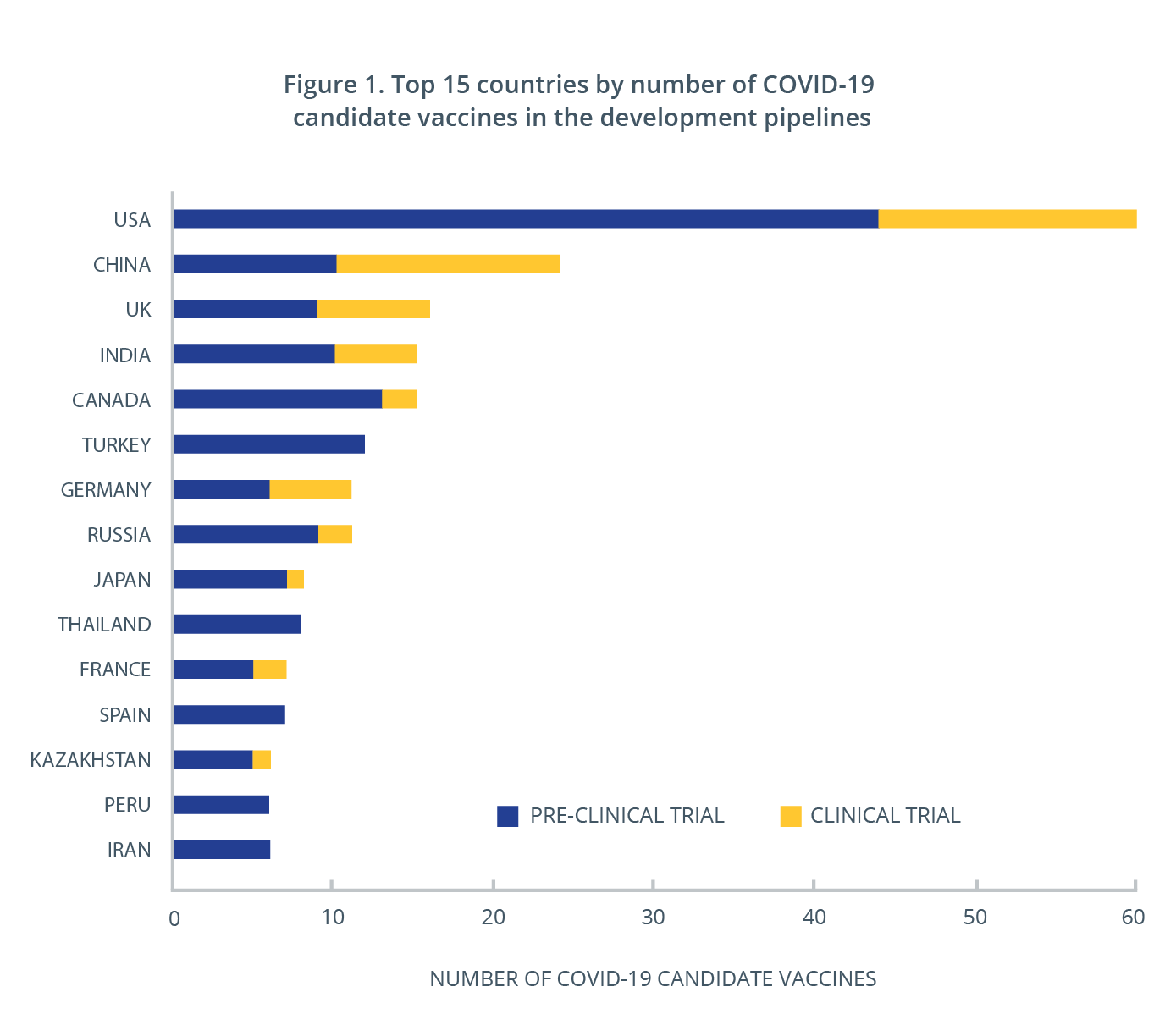
Overall, there are more than 50 candidate vaccines currently in clinical investigation and close to 170 in pre-clinical evaluation around the world. More than 30 countries have been working on developing COVID-19 vaccines, and at least half have one or multiple vaccines in clinical trials (Figure 1). The vaccines expected to be publically available first are the mRNA vaccines from Pfizer/BioNTech and Moderna, which were recently approved for distribution in the U.S., U.K. and Canada. China and Russia have also begun distribution of vaccines they have developed and have chosen to grant authorization ahead of the completion of clinical trials.
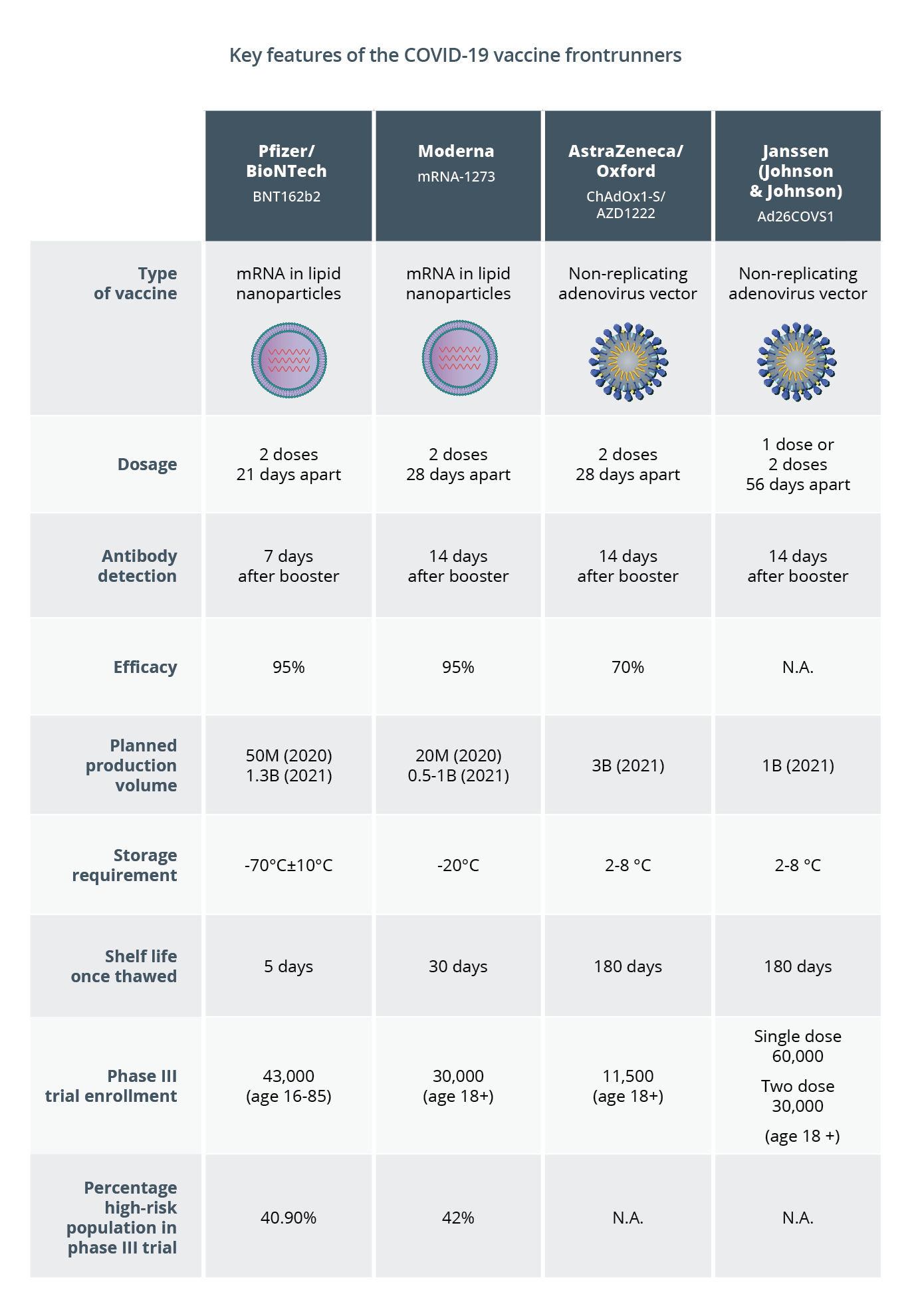
The table below highlights key features of the four leading vaccines for which preliminary efficacy data from late stage clinical trials has been released. The mRNA vaccines both show higher efficacy than the non-replicating viral vector vaccine candidate from AstraZeneca/Oxford does, based on initial data. However, the latter offers some advantages in cost and storage requirements.. Another non-replicating adenovirus vector vaccine developed by Janssen is expected to have large clinical trial enrollment and may only require one dose, but the efficacy data is not available yet.
Keep in mind, however, that the first to market is not necessarily the best long-term solution for everyone. In addition to the above frontrunners, there are many other promising vaccine candidates still working their way through clinical trials. Some of those candidates may offer alternatives to overcome key logistical challenges faced by the leading candidates, including the need for multiple doses, extreme storage temperature requirements, short shelf life and possible low public confidence in new vaccine platforms.
These additional late-stage candidates, if approved, will be especially important to support vaccination programs in places where physical, clinical and public health infrastructure are more limited. For example, NVX-CoV2373, developed by Novavax and currently in phase III clinical trial, is a protein subunit vaccine, based on the same vaccine technology as the existing hepatitis B vaccine. This vaccine candidate appears to have milder side effects, triggers a stronger immune response than the mRNA vaccines, can be stored at normal refrigeration temperatures and may offer public confidence of safety due to familiarity with the technology. Another example, ARCT-021 from Arcturus Therapeutics, currently in phase II clinical study, is another mRNA vaccine candidate. It delivers a self-amplifying RNA payload directly into cells, which requires only one shot at a much lower dose. Thanks to the freeze-drying, ARCT-021 can also be stored at normal refrigeration temperatures.
How do I know that the COVID vaccines are safe?
Vaccination is considered one of the greatest medical achievements of modern civilization. It has freed us from many deadly infectious diseases that decimated populations in the past. Since the pioneering work of Dr. Edward Jenner on a cowpox vaccine more than 200 years ago, vaccines against many deadly infectious diseases such as measles, mumps, rubella, polio, hepatitis A and B, human papillomavirus (HPV) and influenza have become a common part of basic medical care. The development process for the COVID-19 vaccine is no different from that used for the development of these vaccines most people take without concern, other than it has benefited from expedited research and approval focus.
The entire development, evaluation and regulatory process that a vaccine must undergo before being deployed to the public has been carefully designed with safety as the primary focus. Clinical trials of vaccines are massive undertakings that include large groups of diverse volunteers to establish vaccine efficacy and rule out rare short- and long-term safety issues. A wealth of previous vaccine development data shows that most severe vaccine reactions and side effects occur within 6 weeks after vaccination. For this reason, the FDA insists on having at least two months of safety data from a phase III clinical trial before even considering granting EUA, but participant data is tracked for many years to ensure no long-term issues arise.
The clinical trial population sizes for the leading COVID-19 vaccines were consistent with those for past vaccine trials. The Pfizer clinical trial data show that among the 44,000 participants there have been 170 confirmed COVID-19 cases to date. Of those cases, 162 came from the placebo group, while only eight were from the vaccinated group. Moderna’s 30,000-person clinical trial data showed that only five people in the vaccinated group developed confirmed cases of COVID-19, whereas 90 people who received placebo shots became ill. Though most trial participants did experience some side effects, they were mostly mild and included fatigue, headache, injection site pain, and muscle pain, which many participants noted were more significant after the second dose.
How long will the vaccine protect me against COVID-19?
The full extent of immunity for those that have had COVID-19 or gain immunity through vaccination is not yet fully known. Researchers report that the antibody levels in the blood of COVID-19 patients drop quickly during the weeks after their recovery. However, the memory B cells and T cells, both capable of rapid immune response to possible reinfection, are known to last for a much longer period. The idea that these cells may provide longer lasting immunity is supported by the observation that recovered COVID-19 patients are highly unlikely to contract the disease again for at least six months. In addition, scientists also anticipate that COVID-19 vaccine-induced immunity may be stronger than that from natural viral infection. That belief is supported by early antibody tests in people vaccinated with Moderna’s mRNA vaccine that showed higher antibody production than observed in recovered COVID-19 patients as well as longer-term data on many widely used vaccines including human papillomavirus and tetanus vaccines. In addition, from what has been observed to date, this coronavirus does not mutate as fast as the influenza virus, so it is very unlikely that researchers will need to redesign another vaccine for COVID-19 each year.
It is important to understand, however, that most COVID vaccine clinical trials are designed to prove that each vaccine is safe and establish how effectively it prevents disease. They do not necessarily establish that the vaccines prevent infection altogether. Therefore, until further tests are conducted next year, it is still unknown whether these vaccines prevent people from spreading the virus even if they do not become ill. Thus, masks and social distancing may still be recommended until a high portion of the population has been vaccinated.
For more detailed information on COVID-19 vaccines and therapeutics, including scientific insights, open access datasets, and special reports, check out the CAS COVID-19 resources.