Gain new perspectives for faster progress directly to your inbox.
A breakthrough in nanotechnology accelerates vaccine production
While COVID-19 hotspots have continued to emerge driven by the Delta variants, the data still shows that vaccinations are effective at preventing hospitalizations and deaths. While over 4 billion vaccine doses have been administered worldwide, only 27% of the world’s population and only 1.1% of people in low-income countries have received at least one dose of a COVID-19 vaccine. While there are many supply chain challenges for the production and distribution of these vaccines (refrigeration, costs, and transportation), one in particular is the production of lipid nanoparticles for vaccines.
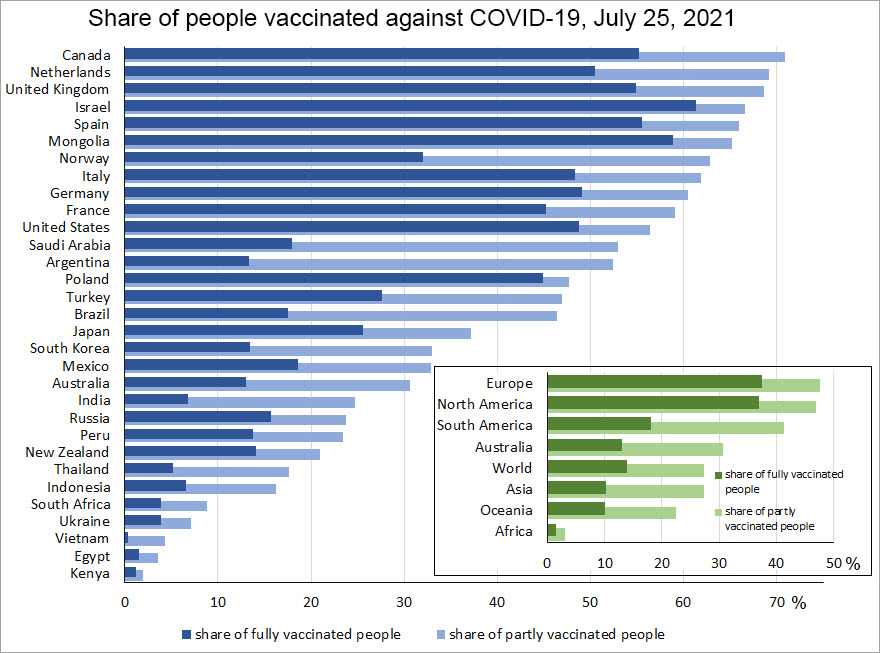
Figure 1: A breakdown by country and continent of the share of vaccinated people
Why are lipid nanoparticles essential for mRNA therapeutics?
Delivery of mRNA therapeutics into the human body has been a major challenge caused by the nucleic acid’s inherent instability and properties:
- The negative charge and hydrophilicity prevent passive diffusion across bio-membranes
- The association with serum proteins, uptake by phagocytes, and degradation by endogenous nucleases obstruct efficient delivery
- Delivery vectors are required to protect them from degradation and to deliver them to the target cells for efficient uptake.
Lipid nanoparticles (LNPs) have proven successful at effectively protecting and transporting mRNA into cells as seen in the recent mRNA COVID-19 vaccines.
Vaccine production is limited by the output of lipid nanoparticles
Scaling the production of any therapy is difficult, but production of these lipid nanoparticles to fulfil the worldwide demand for vaccines is a major challenge. The synthesis of the proprietary ionizable cationic lipids especially developed and optimized for these vaccines is a complex multistep process. But there is even a bigger challenge in producing the LNP on a large scale – the task of combining the lipids and the mRNA into nanoparticles.
Indeed, for efficient production of a pharmaceutical formulation, the manufacturing technique is of utmost importance. Traditional methods for LNP manufacture including but not limited to thin film hydration, reverse phase evaporation, solvent injection, and detergent removal, commonly result in large (>100 nm) and heterogeneous particles with a low encapsulation yield, requiring an additional downsizing step, such as extrusion or sonication. Moreover, these methods are difficult to scale-up and are not consistently reproducible.
Microfluidics is new approach
Recently, microfluidics has proven successful in producing LNPs. In microfluidic focusing method, a stream of lipid solution in alcohol is forced through a channel that is intersected and sheathed by a coaxial stream of an aqueous phase (Figure 2A). Reciprocal diffusion of alcohol and water across the alcohol/water interface causes the lipid to precipitate and self-assemble into LNPs. Microfluidic techniques are robust, scalable, and highly reproducible. For mRNA vaccine formulations, the lipid mixture includes an ionizable cationic lipid, along with a PEG-lipid and helper lipids (phosphatidylcholine, cholesterol), while the aqueous phase contains the nucleic acid. The cationic lipid interacts with the negatively charged nucleic acid, resulting in LNPs with high encapsulation efficiency. LNPs of defined sizes and narrow size distribution can be produced by precisely controlling the microfluidic operating parameters, such as flow rate and component ratios. However, the throughput of the process is limited (<10 mL/h), thus creating a bottleneck for a large-scale production of mRNA vaccines.
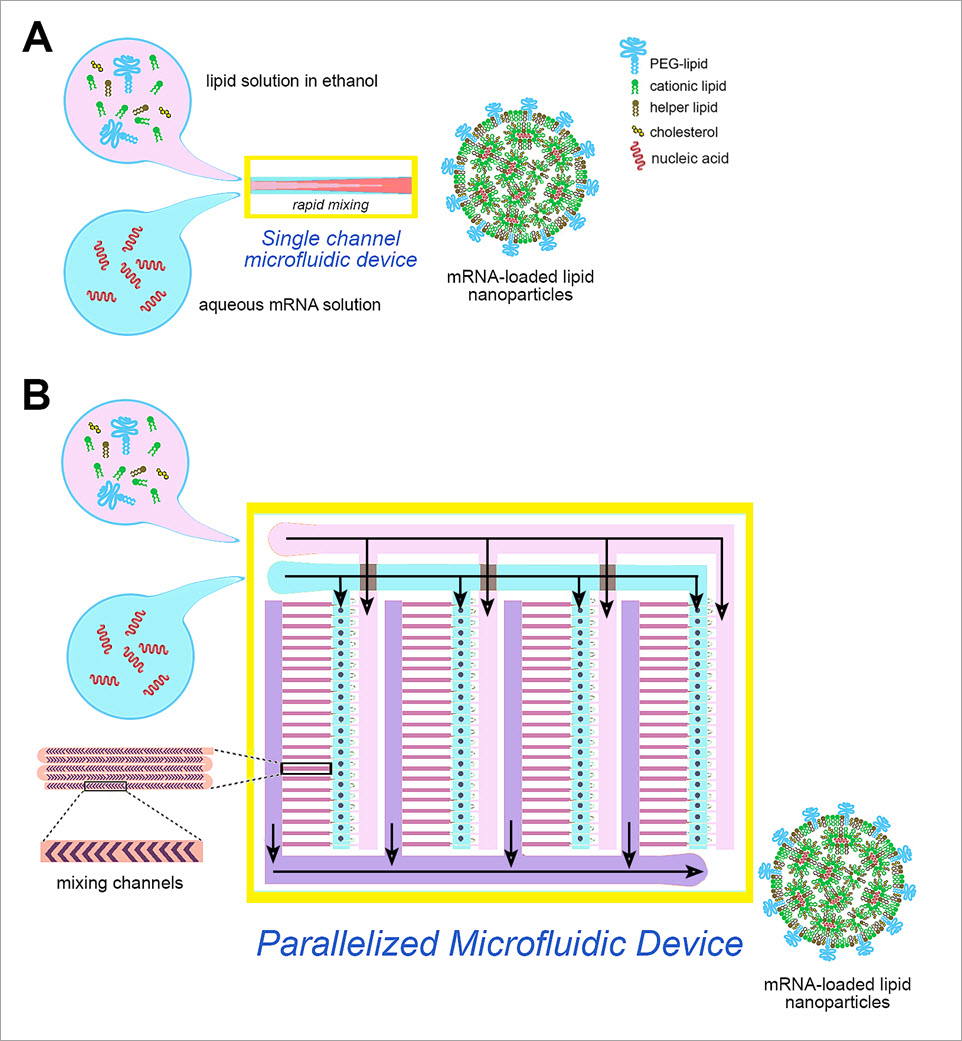
Figure 2. A single-channel microfluidic device (A) and a novel parallelized microfluidic device (B) containing 128 micromixing channels working in parallel
Early results are promising
А recent manufacturing technology breakthrough enabled over hundred-fold increase in the current microfluidic production rates. A microfluidic device has been constructed containing 128 micromixing channels working in parallel – a parallelized microfluidic device, utilizing a very large scale microfluidic integration (VLSMI) platform technology. The channels mix precise amounts of lipid and mRNA, crafting lipid nanoparticles of accurately controlled size and amount of encapsulated mRNA. The device has over hundred times the throughput of a single channel microfluidic device (18.4 L/h) and provides an excellent possibility for even further scaling up, thus allowing mass-production of RNA-carrying lipid nanoparticles. Published results indicate that the parallelized microfluidic device produces lipid nanoparticles effective for use in siRNA- and mRNA-based therapeutics and vaccines.
Lipid nanoparticle production will enable more mRNA therapies
The development of such vaccines and therapies has the potential to revolutionize medicine by gene editing and protein replacement therapeutics. Currently, LNP-based mRNA vaccines have entered clinical trials against a variety of infectious diseases, such as nucleoside-modified mRNA vaccines for Zika virus, cytomegalovirus, tuberculosis, and influenza. mRNA therapeutic vaccines have great potential in cancer immunotherapy against melanoma, ovarian cancer, breast cancer, and other solid tumors.
The use of mRNA for the expression of therapeutic proteins bears great promise in treating a wide range of diseases by applying protein replacement therapy. This newly developed microfluidic fabrication technology addresses the clinical need of scalable, highly precise, and reproducible LNP production, thus enabling rapid formulation of LNPs for a broad range of RNA therapeutics and vaccines. While this may not solely solve the global distribution challenge, it is a critical advancement in a new era of potential cures and vaccines that mRNA may unlock.