Gain new perspectives for faster progress directly to your inbox.
The power of polyethylene glycol
Polyethylene glycol (PEG) is a flexible, non-toxic, hydrophilic polymer with a broad range of applications, from personal care products to pharmaceutical formulations. PEG-lipids have been widely used in pharmaceutical lipid nanoparticle (LNP) formulations for anti-cancer medications such as doxorubicin, irinotecan, and cisplatin, as well as small-interfering RNA patisiran and the messenger RNA vaccines developed by BioNTech/Pfizer and Moderna. The modification of PEGylated pharmaceuticals is a widely implemented approach to reduce clearance by the reticuloendothelial system, extend circulation time, improve pharmacokinetics, and enhance drug efficacy.
However, studies have reported unexpected immune responses against PEGylated nanocarriers. Furthermore, hypersensitivity reactions, including anaphylaxis, have been reported in association with many PEG-containing formulations. This article explores how various structural parameters of the PEG-lipids affect the immune responses and activities of the LNPs regarding their efficiency in drug delivery.
Growing research interest in PEGylated proteins
The global PEGylated proteins market is expected to expand significantly in the next five years, being estimated to reach $2.1 billion by 2028. This growth is largely driven by the rising rate of cancer worldwide, though the technology is increasingly being adopted for other disease areas.
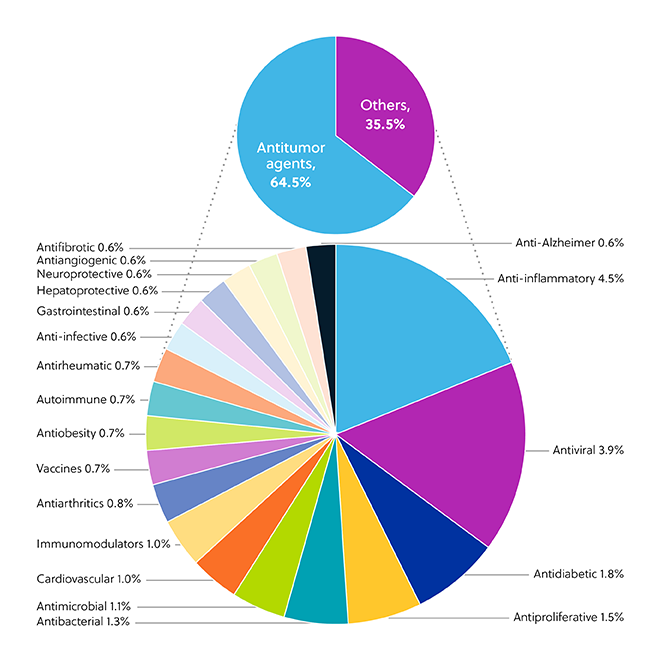
PEGylated LNP formulations are widely explored as therapeutic options against various diseases and disorders and are extensively represented in the CAS Content CollectionTM. While nearly two-thirds of LNP applications (64.5%) are in cancer, other notable targets are anti-inflammatory (4.5%) and antiviral (3.9%) medications (Figure 1).
Polyethylene glycol is considered to have low immunogenicity. Yet, there is growing evidence that it initiates immunogenic responses, especially when conjugated with other materials such as proteins and nanocarriers. Interestingly, anti-PEG antibodies can be found in the general population in individuals who likely have never received systemic PEGylated therapeutics. Furthermore, some PEG-modified compounds induce additional antibodies against polyethylene glycol, which can adversely impact drug efficacy and safety.
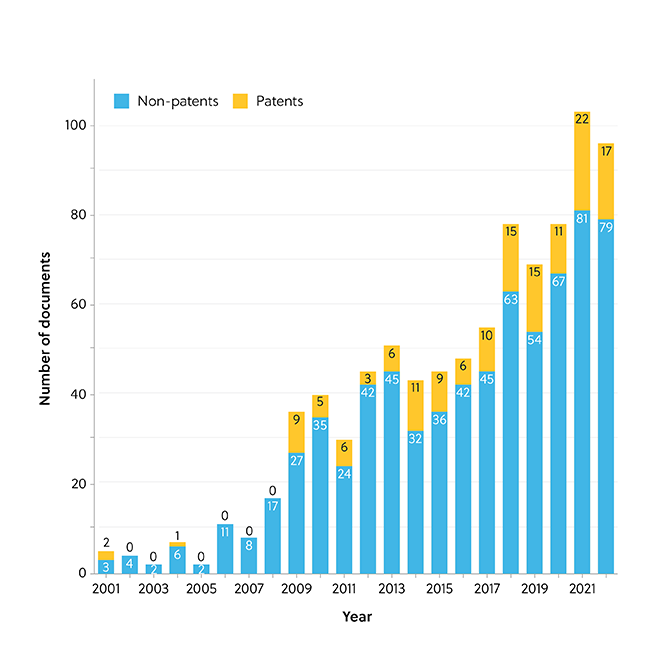
With over 50 million SARS-CoV-2 vaccination boosters administered in the U.S. to date, several questions have arisen about the immunological safety of polyethylene glycol, including PEGylated LNPs. Anaphylaxis was reported to occur in a small number of people (2.5–4.7 per million as of April 2022) shortly after the administration of the Pfizer-BioNTech (Cominarty®) and Moderna (Spikevax®) COVID-19 vaccines. Data from the CAS Content Collection shows yearly growth in the number of documents related to PEG-lipids and their immunologically induced adverse effects up to and including 2021 (Figure 2).
Understanding the immunogenicity of PEGylation
Accelerated blood clearance (dubbed ‘the ABC phenomenon’) is an unexpected immunogenic response observed in PEG-conjugated substances that causes the rapid clearance of PEGylated nanocarriers. The ABC phenomenon has been extensively observed upon repeated administration, resulting in diminished efficacy of PEG-conjugated substances and nanocarriers.
Another unanticipated immune response is a hypersensitivity reaction referred to as CARPA, which significantly reduces the safety of PEGylated nanocarriers and is associated with reduced efficacy of PEGylated therapeutics in clinical trials. The CARPA phenomenon has been classified as a non-IgE-mediated pseudoallergy caused by the activation of the complement system.
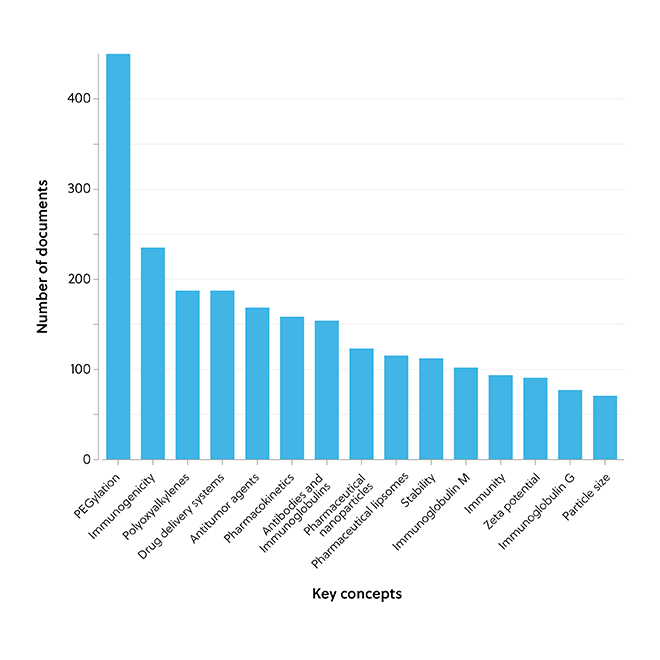
The association between PEGylation and immunologically induced adverse effects of ABC and CARPA is supported by data from the CAS Content Collection, which highlights that PEGylation is a key concept related to these adverse effects (Figure 3).
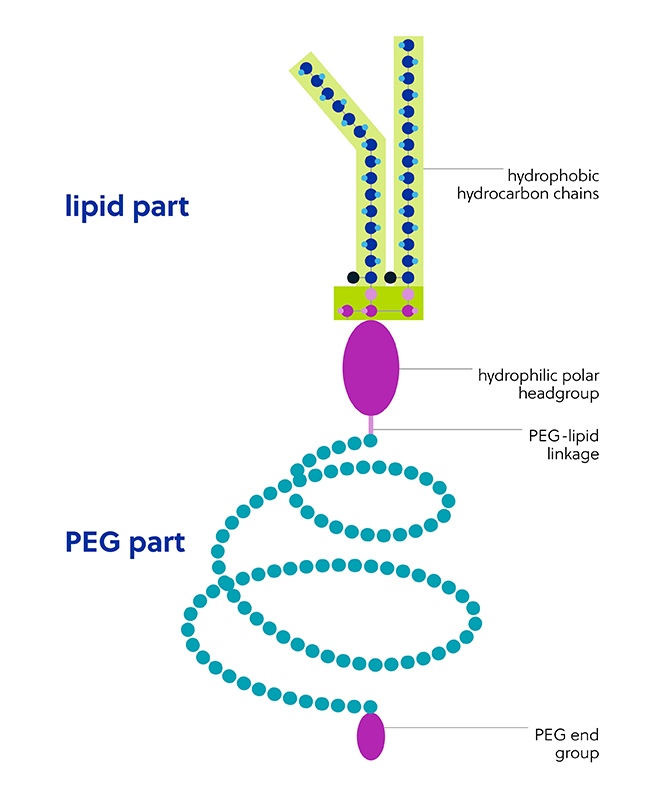
The polyethylene glycol part of the PEG-lipid structure is extremely hydrophilic, flexible, and mobile. Components of the PEG-lipid chemical structure (Figure 4) contribute to the improved stability of LNPs but may also impact their safety and efficacy:
-
PEG length is a key structural factor that impacts immunological safety. The effect appears biphasic, with both long- and short-chain PEG conjugates shown to be more likely to induce the ABC phenomenon.
-
Like PEG length, PEG density (i.e., the percentage of PEG in LNPs) also exhibits a biphasic effect. However, it is both lower and higher densities of PEG that exhibit a reduced ABC phenomenon.
-
Differences in PEG architecture can have an impact, with branched PEG-lipid conjugates conferring higher stealth properties to LNPs than linear PEGs.
-
Functional terminal groups appended to PEG particle chains are an additional factor that impacts their immunogenicity and rate of clearance.
-
Parameters such as size and surface charge can also impact immunogenicity. For example, PEGylated carriers comprising negatively charged phospholipids can stimulate the immune system via complement activation to a greater extent than uncharged vesicles.
-
Like the PEG part, the lipid hydrophobic chain structure and length can also determine the extent of immunogenic effects, but also efficacy.
-
The specific lipid anchoring group employed (e.g., cholesterol as the anchoring group) yields PEGylated LNPs with longer permeance in the circulation and higher systemic bioavailability.
-
Lipid linkage is an important parameter in lipid design and performance, where the substitution of an ester linkage for a carbamate linkage allowed for the formation of unstable vesicles.
Enhancing the safety and efficiency of polyethylene glycol in medicines
While PEGylation has become a gold standard in pharmaceutical nanocarrier modification for developing successful drug delivery systems, immunosafety is a key issue in current research and development of nanomedicines such as LNPs. In fact, there are currently more than 200 clinical trials registered on ClinicalTrials.gov to examine PEGylated lipid safety, mainly PEGylated liposomal doxorubicin in various solid tumors and the mRNA SARS-CoV-2 vaccines, Comirnaty®, and Spikevax®.
Understanding the factors that impact anti-PEG antibody production is crucial for both researchers and clinicians to develop novel drug vehicles or to adjust the route of administration and injection schedule to ensure the highest treatment efficacy.
An array of alternative polymers, such as poly(oxazoline), polyvinyl alcohol, and poly(glycerol), have been investigated to overcome immunogenic issues with polyethylene glycol. Despite proven benefits, no agent has yet been proven to be superior to PEG in terms of augmenting the pharmacokinetic performance of LNPs, and they carry their own hypersensitivity risks. Other alternative polymers that mimic the stealth properties of polyethylene glycol, including zwitterionic and hydrophilic polymers, are currently in development.
Though recent research has helped elucidate many of the factors that contribute to immunogenicity, the immune toxicology of nanomedicines is a largely unexplored research area at a broad intersection of nanotechnology, immunology, and pharmacology. Improved knowledge in this area can enable us to develop optimal pharmaceutical formulations that diminish undesirable immune reactions and enhance the safety and efficiency of PEGylated medicines.
Read our Executive Summary or our in-depth, peer reviewed journal publication in Bioconjugate Chemistry.