Gain new perspectives for faster progress directly to your inbox.
The angiotensin-converting enzyme 2 (ACE2) protein has drawn considerable attention in recent years for its role as a receptor of the SARS-CoV-2 virus, but the flurry of research into ACE2 has also revealed intriguing possibilities for it as a therapeutic target in a number of other diseases.
What is ACE2?
ACE2 is a membrane protein with an enzymatic domain located on the outer surface of human cells. It was so named because this protein was initially identified as a homolog (or a variant) of angiotensin-converting enzyme (ACE), an enzyme that mediates the formation of the peptide hormone, angiotensin II from angiotensin I. ACE has been studied extensively and is a well-known vasoconstrictor (i.e., it causes muscle contraction in the blood vessel wall and narrowing of the blood vessel lumen).
ACE2, now known to be a viral receptor, also acts as a vasodilator, which counterbalances ACE and causes blood vessel walls to relax. Both ACE and ACE2 are important players in the renin-angiotensin system (RAS) that regulates blood pressure and blood flow to multiple organs, including the lungs, heart, and kidneys.
Functions of the angiotensin-converting enzyme 2
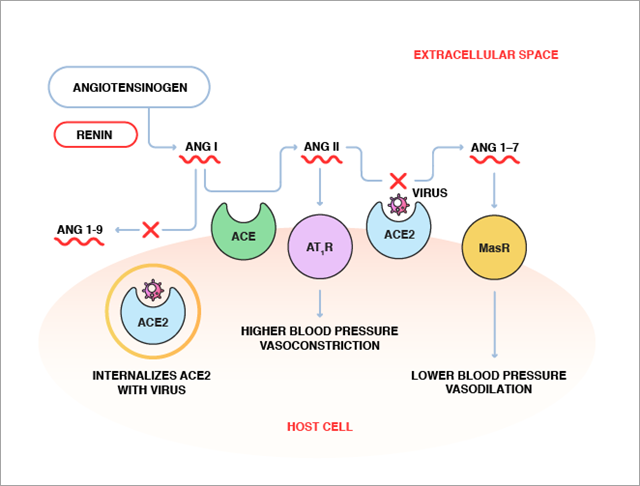
The renin-angiotensin system encompasses a complex network of enzymes, peptide hormones, and receptors, as shown in Figure 1. Angiotensinogen, the angiotensin (Ang) precursor, secreted by the liver, is cleaved by the kidney enzyme renin to produce Angiotensin I (Ang I). Ang I is then converted to Ang II by ACE. Ang II, an eight-amino acid hormonal peptide, binds to type 1 angiotensin receptors (AT1R) on the surface of muscle cells in small blood vessels to cause vasoconstriction. It also promotes sodium reabsorption by the kidneys. Both vasoconstriction and sodium reabsorption lead to an increase in blood pressure. Thus, abnormally high ACE activity leads to increased levels of Ang II, causing hypertension.
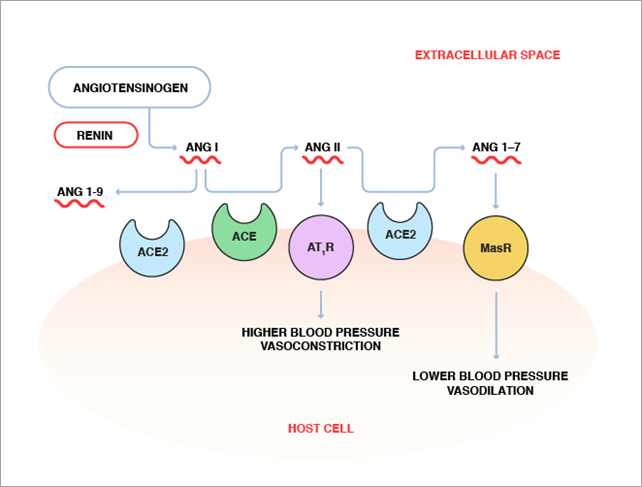
Conversely, ACE2 catalyzes the conversion of the eight-amino acid peptide, Ang II, to a seven-amino acid peptide (Ang 1-7), which appears to have the opposite effect of Ang II via its action on a different receptor, called Mas receptor (MasR). While the precise role of Ang 1-7 in blood pressure regulation has not been fully elucidated, evidence exists that it lowers blood pressure and induces vasodilation. In addition, ACE2 cleaves Ang I to Ang 1-9, and therefore may further counterbalance the effect of ACE by removing its substrate. By causing the conversion of Ang II to Ang (1-7) and Ang I to Ang 1-9, ACE2 may play a role in maintaining the balance between vasoconstriction and vasodilation to keep blood pressure in check.
The role of ACE2 in SARS-CoV-2 infection
Since the outbreak of COVID-19, scientists have been racing to understand the SARS-CoV-2 virus, elucidate the mechanism for disease progression, and identify treatment options. Extensive research has been devoted to identifying viable genes and proteins as targets for therapeutic agents and, early in the pandemic, a potentially important role for ACE2 as a receptor for the the SARS-CoV-2 virus was discovered.
ACE2 can be recognized by the spike protein (S protein) on the surface of the SARS-CoV-2 or SARS-CoV virus. ACE2 and S protein bind in a fashion analogous to a lock-and-key interaction, which enables the virus to enter human cells (Figure 2).
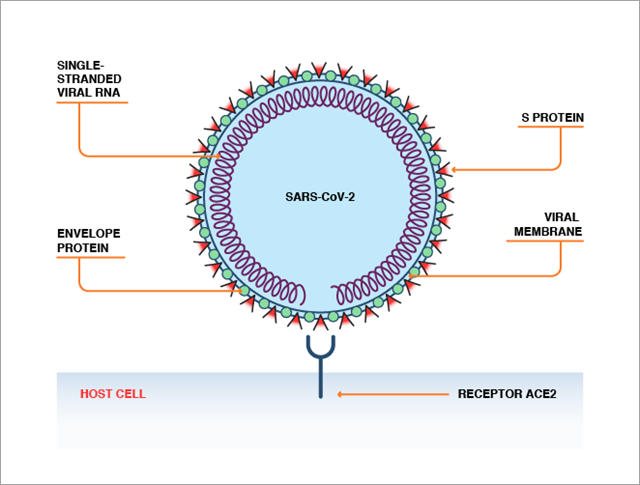
Although SARS-CoV-2 is very similar to SARS-CoV, the virus that caused SARS (severe acute respiratory syndrome), a few mutations in the receptor binding domain of the S protein have significantly increased the SARS-CoV-2 virus’ binding affinity to ACE2. These differences may underpin the higher transmissibility of COVID-19. There is evidence that ACE2 is expressed in our lungs, digestive systems, hearts, arteries, and kidneys. ACE2 expression also increases with age and is higher in patients suffering from cardiovascular diseases, potentially explaining the increased severity of COVID-19 in these subgroups.
ACE2 protein interactions in COVID-19 therapies
While functioning as the docking site for SARS-CoV-2 and mediating virus entry into the host cells, ACE2 may not act alone in this process. Other host enzymes are also involved in facilitating viral entry. Enzymes called proteases are responsible for removing fragments both from ACE2 and S proteins to enhance their interaction process. Other enzymes modify the ACE2-S protein complex packed in membrane-bound vesicles to facilitate viral entry into the host cell. Therefore, ACE2 and its interaction with SARS-CoV-2, as well as other proteins involved in this process, are conceivably valid targets for anti-COVID-19 agents.
Upon viral binding, it is speculated that the catalytic domain of ACE2 may be blocked by the virus, resulting in limited access to the substrate, Ang II, causing Ang II accumulation. Additionally, with viral entrance, the surface ACE2 may be internalized to the cells, therefore decreasing ACE2 enzymatic function (Figure 3). As a result of reduced ACE2 activity, circulating Ang II levels may increase, as has been reported in COVID‐19 patients. The Ang II level exhibits a linear positive correlation to viral load and lung injury, indicating a direct link between tissue ACE2 downregulation with RAS imbalance and the development of organ damage in COVID-19 patients. More studies, however, are needed to confirm this finding.
The potential of ACE2 as a target for COVID-19 therapies
Due to the crucial role ACE2 plays in host cell invasion by SARS-CoV-2, efforts are underway to develop drugs that can block its function in this capacity. To date, no small-molecule drug has been approved via drug repurposing for this application. However, a recently developed biologic drug may achieve this goal. This clinical-grade drug, human recombinant soluble ACE2 (hrsACE2), was originally designed for acute respiratory distress syndrome (ARDS).
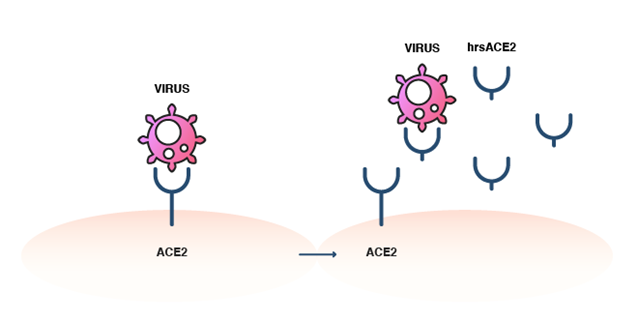
The hrsACE2 does not have the membrane-attachment segment and, thus, does not attach to human cells. However, it is capable of binding to the SARS-CoV-2 virus as a decoy receptor. By competitively binding to this coronavirus, it prevents viral binding to the natural, membrane-bound ACE2 and, thus, blocks virus entry into host cells (Figure 4). Studies in cultured cells and various organoids have indeed shown that hrsACE2 inhibited the virus from infecting the host cells. It also appeared to be well tolerated and elicited a rapid decrease in serum Ang II levels in ARDS patients in a 2017 clinical trial. It is hopeful that hrsACE2 may be the first drug that targets ACE2 and will open the door for targeted therapies in the fight against COVID-19. Encouragingly, hrsACE2 has shown potential as a combination treatment, by improving the effectiveness of remdesivir in SARS-CoV-2 infection.
Future therapeutic applications of ACE2
Beyond Covid-19, the ACE2 pathway offers a potential route to treat other respiratory diseases, such as novel 2009 influenza (H1N1) and avian influenza (H5N1), possibly by developing recombinant ACE2 for use along with an AT1R inhibitor or ACE inhibitor. Cardiovascular diseases are another area where ACE2 is of growing interest, and novel targets like ACE2 could help find more effective ways to target the RAS hyperactivity that plays such an important role in conditions like hypertension. ACE2 is also likely to be an important target in the fight against Type 2 diabetes, for example, by using ACE2-mediated pathways to negate the effects of overactive Ang II in the diabetic kidney.
The emerging role of RNA in the development of novel therapeutic treatments is reshaping the landscape of drug discovery, so stay ahead with CAS. Explore our Insight Report on the emerging landscape of RNA therapeutics.