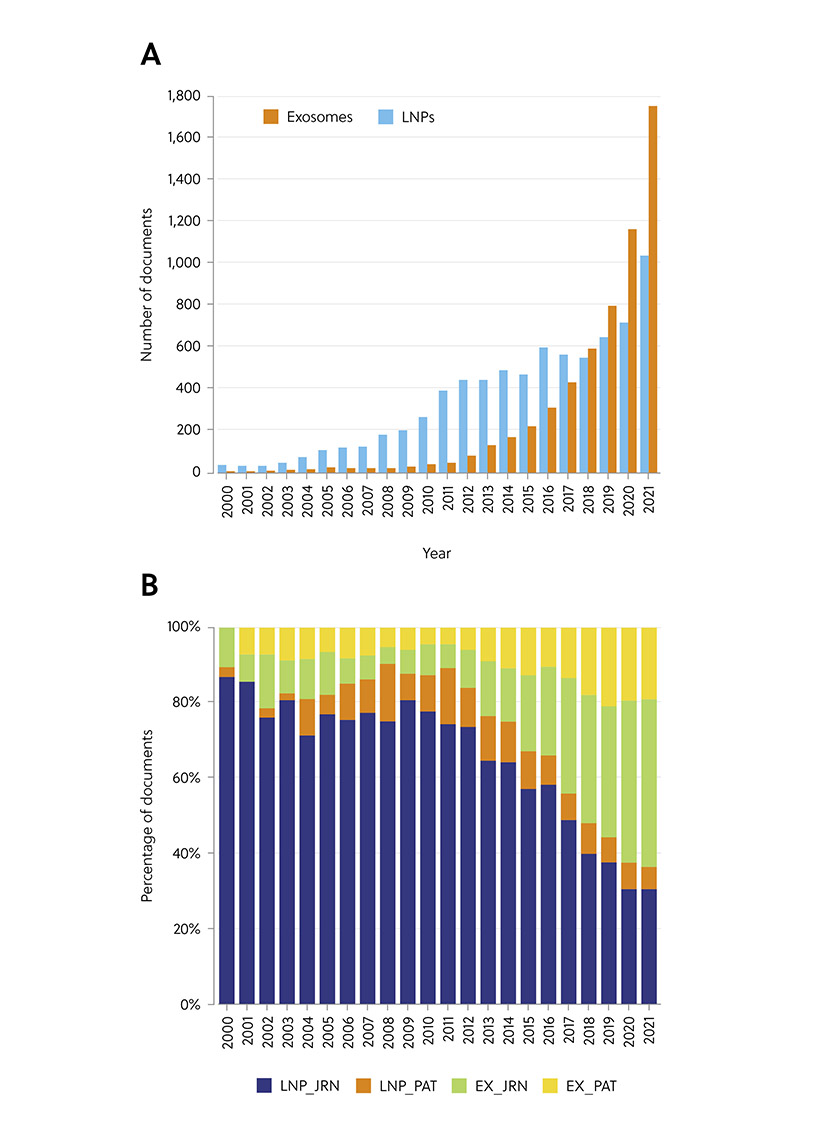
Gain new perspectives for faster progress directly to your inbox.
In collaboration with National Natural Science Foundation of China and National Science Library at the Chinese Academy of Sciences, this detailed landscape of synthetic organic chemistry was published jointly in Organic Letters