Gain new perspectives for faster progress directly to your inbox.

Cytokine storm warning: the critical role of cytokines in immunity and infection
The leading cause of death associated with COVID-19 is respiratory failure, followed by septic shock, heart failure, hemorrhage, and renal failure. Serological tests indicate that inflammatory cytokine storm response is associated with COVID-19 severity and mortality. Therefore, enhancing our understanding of the biological function of cytokines and the underlying mechanisms of the cytokine storm response in some COVID-19 patients is key to developing effective treatment strategies and reducing the mortality rate.
What is a cytokine?
Cytokines are a group of low-molecular-weight extracellular signaling proteins produced by various immune cells including macrophages, lymphocytes, and mast cells, as well as other cell types such as endothelial cells (cells that form the interior lining of blood and lymph vessels). There are several classes of cytokines in the human body including interleukins (IL), interferons (IFN), lymphokines, chemokines, and tumor necrosis factors (TNF). Cytokines act as immune modulators in our bodies by binding to cell surface receptors responsible for regulating various biological functions including cell proliferation and innate and acquired immune responses, as well as pro- and anti-inflammatory actions.
During viral infections, cytokines stimulate our immune system to eliminate pathogens, remove destroyed cells, and repair injured tissue. In this way, cytokines help our body fight pathogens. However, when unbalanced or overproduced, they can also have severe adverse effects.
How will RNA therapeutics reshape the landscape for infectious diseases and viral infections? Learn more in our latest Insights Report on the emerging trends and future opportunities for RNA
Causes of cytokine storm disorders
Cytokine storm syndrome (CSS) or cytokine release syndrome (CRS) is a systemic inflammatory response commonly caused by viral infections. It is characterized by many cells releasing excessive amounts of pro-inflammatory cytokines. This uncontrolled inflammatory process can cause septic shock, multi-organ damage, and even organ failure.
Sometimes when a virus enters human host cells, it replicates and releases progeny viruses, and this may cause pyroptosis (programmed cell death triggered by inflammation), which activates our innate and adaptive immune systems. The viral infection triggers epithelial cells and alveolar macrophages in the lungs to produce various inflammatory cytokines and chemokines, as shown in Figure 1. These forms of cytokine storms attract monocytes, macrophages, and T cells to the infection site which produce more inflammatory cytokines, forming a feedback loop. Aggregation of T cells in the lungs also causes a decrease in the levels of lymphocytes in the blood (i.e., lymphopenia) in severe COVID-19 patients.
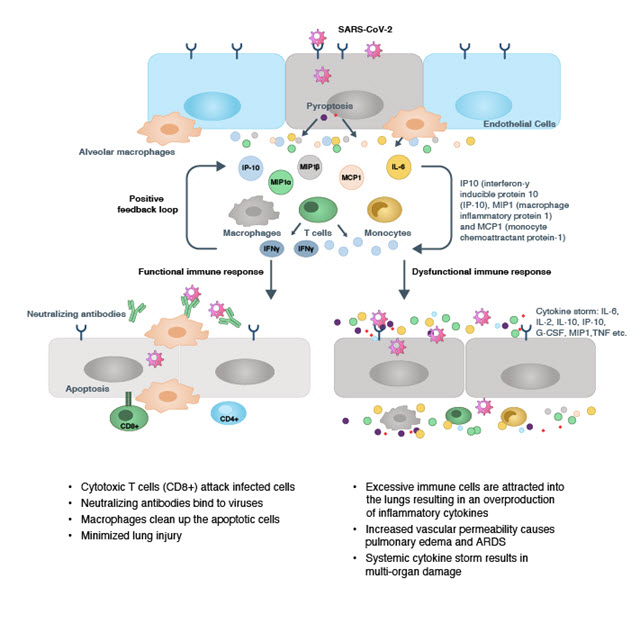
The body’s response to cytokine storms
The initial activation of immune cells triggers either a functional or dysfunctional immune response. During a functional immune response, cytotoxic T cells (CD8+) directly attack the infected cells, while neutralizing antibodies bind the virus and initiate cell death (apoptosis). Then, the lung alveolar macrophages clean up neutralized viruses and engulf the apoptotic cells, a process called phagocytosis. In most cases, infections are resolved through this process; the level of inflammatory cytokines recedes, and patients recover.
In some cases, however, a dysfunctional immune response attracts additional immune cells to the lungs. This results in the overproduction of inflammatory cytokines, leading to a cytokine storm. During a cytokine storm, an increase in vascular permeability allows fluid and blood cells to move into the lung alveoli, resulting in pulmonary edema, ARDS, and even respiratory failure. These clinical manifestations of cytokine storm can also cause systemic inflammation such as sepsis and disseminated intravascular coagulation, tissue damage, and eventually multi-organ failure.
Lymphopenia and elevated cytokine profiles exhibit a positive correlation with virus titers and disease severity. Thus, such serological measurements could help physicians effectively identify patients who are susceptible to cytokine storm syndrome and provide timely intervention. However, more studies are needed to fully define the role of cytokines and the associated pathophysiological mechanism of the host immune response in severe COVID-19.
Therapeutic strategies to weather the cytokine storm
Currently, there are no approved drugs for managing the onset of cytokine storms in severe COVID-19 cases. Although corticosteroids are commonly used to inhibit inflammation, such drugs should be used with caution for COVID-19 patients and may worsen associated lung injuries. Alternative immunosuppressant strategies targeting several cytokines and their receptors are under investigation, including those that target the IL-6-mediated pathway.
SARS-CoV-2 infection activates immune cells and releases IL-6 and other inflammatory cytokines. As shown in Figure 2, IL-6 then binds to the soluble IL-6 receptor (sIL-6R), forming a complex with another protein, gp130 dimer, on the surface of endothelial cells. This triggers the release of cytokines from endothelial cells, which in turn attract immune cells to the infection site and produce more cytokines resulting in a cytokine storm. IL-6 receptor antagonists bind to the IL-6 receptors and block their interaction with IL-6 as well as the subsequent biological events.
Although excessive IL-6 can cause cytokine storms in COVID-19 patients, IL-6 also plays an important role in their recovery by supporting lung repair and remodeling. As such, could also be a critical factor affecting patient outcomes.
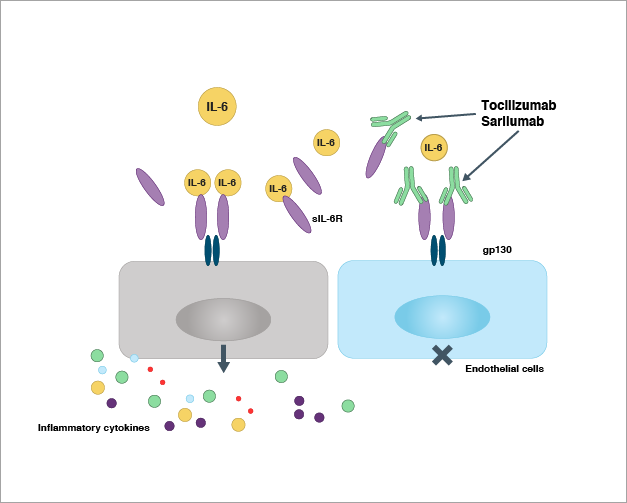
Ambiguous results from cytokine storm clinical trials
A clinical trial of tocilizumab, an anti-IL-6 receptor antibody approved for treating cytokine storm syndrome associated with some cases of CAR-T cell therapy, was initiated in patients with COVID-19 in China. Initial results from 21 severe patients treated with tocilizumab are promising. Body temperature returned to normal on the first day of treatment in all patients. 75% of patients required less oxygen support, and all patients were eventually discharged. Tocilizumab also underwent clinical trials in the USA, results showed it did not improve mortality but was associated with shortened hospital stays. Sarilumab, another anti-IL6R antibody, also underwent clinical testing and showed no significant efficacy in treating cytokine storm syndrome.
While these results are disappointing, cytokines constitute an important host defense system and modulate immune responses, and they may also contribute to the development of severe cases of COVID-19 and even lead to death. Therefore, the development of therapies to effectively control the cytokine storm is a key research focus necessary to reduce COVID-19 mortality and may advance our understanding of the role of cytokines in other inflammatory responses.
Explore how therapeutic innovations will revolutionize medicine beyond COVID-19 for generations in the CAS white paper: “RNA-Derived Medicines: A review of the research trends and developments”



 Download the case study now
Download the case study now