Gain new perspectives for faster progress directly to your inbox.
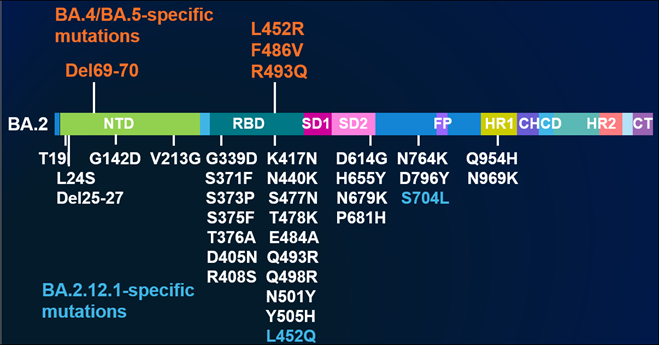
Recently, the World Health Organization (WHO) classified a new SARS-CoV-2 variant of concern. The new variant, designated Omicron (B.11.539), was first identified by a robust genetic sequencing network in South Africa and reported to the WHO. This unique variant is the most heavily mutated to date, including more than 50 identified mutations, more than 30 of which are on the spike protein.
Why is the Omicron variant so concerning?
Preliminary study of the Omicron variant indicates that it has evolved significantly from the original version of the virus first identified in China in 2019, which means there is an increased likelihood that it could reinfect those who have already had COVID-19 or evade the immunity generated by the current first generation of vaccines. Such a variant could drastically set back the global pandemic recovery, resulting in staggering societal and economic impacts.
Its structure is the primary reason the Omicron variant brings about greater concern over immune evasion and transmissibility, even amongst vaccinated individuals. Its multiple receptor-binding domain (RBD) and N-terminal domain (NTD) mutations are associated with resistance to neutralizing antibodies, and protease cleavage mutations are expected to enable easier cell entry or increased transmission. In fact, several studies have shown these mutations affect the immune system's ability to neutralize the mutated SARS-CoV-2 virus and may even reduce immune neutralization tenfold.
The new variant was originally found in South Africa with cases now identified in Australia, Europe, Canada, Asia, and just recently in the United States. However, many experts believe, based on the previous experience with the spread of the Delta variant, that Omicron has already traveled over much of the globe. Although there have been small molecule drugs, e.g., nafamostat, camostat mesylate, which target non-spike proteins of SARS-CoV-2 and will probably remain effective for Omicron, developing a sustainable vaccine for the current and future variants are crucial for ending this pandemic.
The role of spike protein
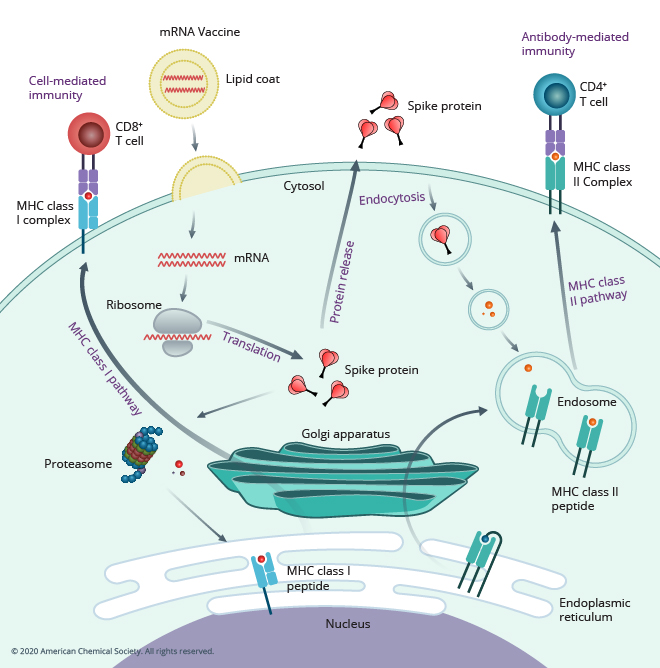
In summary, the SARS-CoV-2 viral spike (S) protein, as shown in the figure below, is the key antigen targeted by the first generation of COVID-19 vaccines, because it enables the SARS-CoV-2 virus to enter human cells. Today’s current mRNA vaccines neutralize the S protein by encoding it as the antigen.
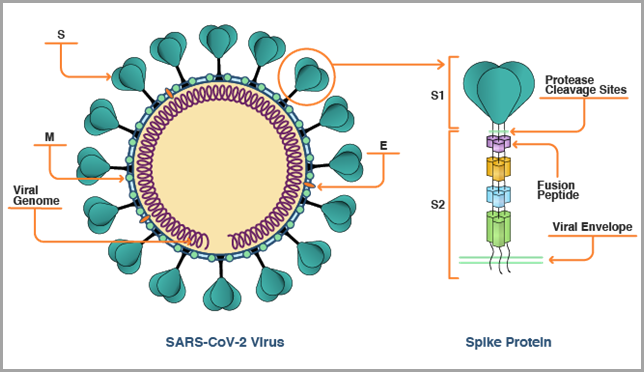
The rapid evolution of the SARS-CoV-2 virus is forcing researchers to assess if the S protein alone is an adequate antigen for vaccine design. With an initial portfolio of vaccines and therapeutics now being deployed to blunt the pandemic, scientists must now consider all that has been learned over the last two years about our immune response to the SARS-CoV-2 virus, what responses correlate with protective immunity, and what relevance these studies have to the construction of second-generation vaccines that potentially will need to expand on or move away from targeting the ever-mutating S protein.
The path to achieving sustained immunity
Many factors determine how humans develop “protective immunity,” especially against an evolving and mutating virus, like SARS-CoV-2. Ideally, we’d like to achieve an immune response that can recognize and disable things like invading viruses and bacteria. Protective immunity includes both humoral and cellular immunity. Immune responses to SARS-CoV-2 reported in some recently published studies are shown in the table below. Antibody and T-cell responses to COVID-19 proteins in people who survived infection appear broad, but not complete. These responses primarily target virus structural proteins (S, nucleoprotein, membrane protein). A subset of these responses is likely responsible for protective immunity.
| Humoral immunity | Cellular immunity | ||||
| Protein antigen | IgG* | IgA* | CD4 T-cell | CD8 T-cell | Memory T-cell |
| 3C-like protease | + | + | |||
| S protein | + | + | + | + | + |
| Nucleoprotein | + | + | + | + | + |
| Envelope small membrane protein | + | ||||
| Membrane protein | + | + | + | + | |
| Orf3 protein | + | + | |||
| Orf6 protein | |||||
| Orf7a protein | + | ||||
| Accessory protein 7b | + | ||||
| Orf8 protein | + | + | + | ||
| Orf10 protein | |||||
*Includes neutralizing antibodies against S protein
The S protein antigen covers the surface of the virus, making it easy for the immune system to find, and an ideal target for protective immunity. However, some humoral and cellular immune responses shown in the table above target SARS-Cov-2 antigens other than the spike protein. These observations, along with the emergence of viral variants and breakthrough infections, suggest that a vaccine focused on immune responses against a single antigen (i.e., the S protein) may not provide the broad immunity needed for an effective vaccine. These types of neutralizing antibodies are often a necessary part of protective immune responses but are not sufficient to establish durable immunity. Whether humoral and cellular responses against other SARS-CoV-2 antigens provide broadly protective immunity needs to be further studied.
What lessons can we learn about achieving sustained immunity from influenza vaccines?
Although we don’t fully understand protective immunity in SARS-CoV-2 infection, recent studies of the influenza virus (IAV) vaccine provide some useful insights. Immunization with the IAV matrix protein (M2e) or nucleoprotein (NP) both produce protective immunity. COVID-19 vaccine researchers could try targeting both the highly variable SARS-CoV-2 S protein and a unique, conserved, SARS-CoV-2 protein, such as ORF8. While the ORF8 protein is currently uncharacterized, it may be a new, conserved antigenic target for second-generation SARS-CoV-2 vaccines.
A small number of current clinical and preclinical vaccines use whole inactivated virus (IV) or live-attenuated virus (LAV), which should present a broad variety of antigens to the immune system. An even smaller number of multiepitope/multiantigen vaccines are in development and clinical data is lacking. While preliminary suggests that acceptable levels of protection could be achieved, the causes of reduced neutralizing antibodies are not clear and further research is needed to better understand protective immunity.
An evolving virus requires new treatments
Current vaccines against SARS-CoV-2 that solely target the S protein are an important initial step toward developing antiviral therapeutics and next-generation vaccines. The speed and efficacy with which these were created were unprecedented. Even with new variants like Delta, the data shows that vaccines have drastically reduced hospitalizations and deaths. However, as the virus continues to mutate and evolve, solely targeting the S protein may not be enough for protective immunity. We expect a more comprehensive approach that may include several types of vaccines combined with antiviral therapies that target a broader range of antigens truly stopping the viral spread.
Want to stay up to date on the latest COVID-19 information and resources? Visit our COVID-19 Resources page for unique insights, peer-reviewed publications, and data to accelerate your COVID-19 research. Also, be sure to subscribe to the CAS blog for ongoing updates and insights.