Gain new perspectives for faster progress directly to your inbox.

Ammonium nitrate (AN) is a widely used chemical compound with several important applications. As a fertilizer, it helps feed billions. It is also the main component in many types of mining explosives, where it is mixed with fuel oil and detonated by an explosive charge. Increased global agriculture activities, combined with the rising demand for ammonium nitrate fuel oil (ANFO), mean that the market for ammonium nitrate is expected to grow at a compound annual growth rate (CAGR) of at least 4% over the next 5 years. However, the potentially explosive nature of ammonium nitrate (and the potential for its misuse) means that manufacturers, sellers, and users of this compound need to heed safety warnings or risk disaster.
How can we continue to reap the benefits of this versatile compound while minimizing the risks associated with its use? In this article, we will discuss the key learnings from the catastrophic 2020 ammonium nitrate explosion in Beirut and how further explosions can be prevented.
Ammonium nitrate – a market on the rise
The ammonium nitrate market is on an upward trajectory, with recent reports estimating a market size of $24 billion by 2026. This increase in market value has been catalyzed by the growing population, creating an increased demand for food supply and real estate. Incidentally, ammonium nitrate is a valuable resource for both industries.
Ammonium nitrate is a popular fertilizer since nitrogen is a key component of the two parts of the compound: NH4 (ammonium) and NO3 (nitrate). Not only can plants access nitrogen directly from the nitrate form of the compound, but the ammonium fraction can also be gradually converted to nitrate by soil microorganisms. These properties make ammonium nitrate a popular choice for vegetable growers, who prefer a readily available nitrate source for plant nutrition. Animal farmers use ammonium nitrate for pasture and hay fertilization, preferring it to urea-based fertilizers that can volatize from the soil before it is used by the plants. It is also highly soluble, making it well- suited for use in irrigation systems.
Ammonium nitrate's other predominant use is as a component in explosive mixtures used for mining, quarrying, and civil construction purposes. As part of ANFO, it accounts for 80% of the explosives used in North America. Unfortunately, as the components of ANFO are relatively easy to obtain, there is a potential for misuse in improvised explosive devices. This highlights the importance of proper control of this ammonium nitrate form to reduce its potential hazards.
Beirut disaster – the aftermath
On August 4, 2020, the capital of Lebanon, Beirut, was shaken by a catastrophic explosion that killed at least 218 people and left over 6,000 people injured. The main cause of the explosion was approximately 2,750 tons of ammonium nitrate stored in a warehouse at the Port of Beirut. This large amount of ammonium nitrate had been seized from an abandoned ship impounded in 2014. The stored fertilizer was ignited by sparks from a fire at an adjacent warehouse, creating an explosion that caused enormous property damage and left an estimated 300,000 people homeless. Two years on, the explosion continues to impact Beirut, with subsequent collapses of adjacent grain silos occurring in July and August 2022.
Aside from the human cost of the explosion, the economic impact is thought to be in excess of 6.7 billion US dollars. The explosion wiped out 90% of Lebanon’s grain reserves, adding to an already precarious food security situation in a country facing severe economic challenges. The environment must also have been affected by such a blast, although data is lacking. When the ammonium nitrate exploded, noxious gases including nitrogen oxides, ammonia, and carbon monoxide were released into the environment, causing chemical pollution and further harm to local people. Ecosystems are also damaged by this environmental pollution, with amphibians and aquatic life bearing the brunt of nitrate poisoning as the decomposition products transfer to the ocean.
Experts have analyzed the Beirut explosion and compared it to other similar ammonium nitrate disasters. In common with several other devastating explosions, the root cause of the explosion was deemed to be an uncontrolled fire. The stockpiling of such a large quantity of ammonium nitrate in one location amplified the impact of the explosion, and the urban nature of the storage location increased the number of blast injuries that resulted. Because of these analyses, recommendations have been made including initiating a Chemical Regulatory Agency to take control of chemical safety at a national level in Lebanon and improving planning for emergency responses to future events.
The explosive nature of ammonium nitrate
Most ammonium nitrate explosions take place during transport or storage (Figure 1), however to fully understand the risk factors for an explosion, it is important to appreciate ammonium nitrate’s chemistry and manufacturing processes.
Ammonium nitrate is made by the reaction of ammonia with nitric acid in water followed by careful evaporation of the water to yield a solid element:
NH3 + HNO3 → NH4NO3
Ammonia is usually derived from atmospheric nitrogen and nitric acid is prepared from the combustion of ammonia. These two starting products would not commonly be kept near one another. Manufacturing occurs in an aqueous solution because the reaction produces a significant amount of heat. The subsequent evaporation process has been the source of several explosions. Other sources of explosions relating to the manufacturing process include the inclusion of impurities, which can lower the stability of ammonium nitrate. Without adequate temperature control, it is also possible for ammonium nitrate to absorb water or change crystal forms leading to agglomerates that make it unsuitable for use.
Despite decades of study into the reactions of ammonium nitrate, the exact mechanisms of decomposition and explosion are not fully understood. This conundrum is in part due to the chemical complexity of the reaction but also the varying ambient conditions and potential contaminants. The following reaction has been hypothesized as the main detonation reaction:
2NH4NO3 → 2N2 + O2 + 4H2O
One reason that ammonium nitrate is so explosive is that it contains in the same molecule both a fuel, in the form of the ammonium ion, and a strong oxygen- producing agent, nitrate. As decomposition occurs, heat is produced which initiates detonation and, because an oxygen source is already present, combustion accelerates rapidly. The result is the production of nitrous oxides, oxygen, water, and large amounts of heat and kinetic energy. These products cause an expansion in volume 1,000 times greater than the initial volume of ammonium nitrate, leading to catastrophic blast damage in the surrounding area.
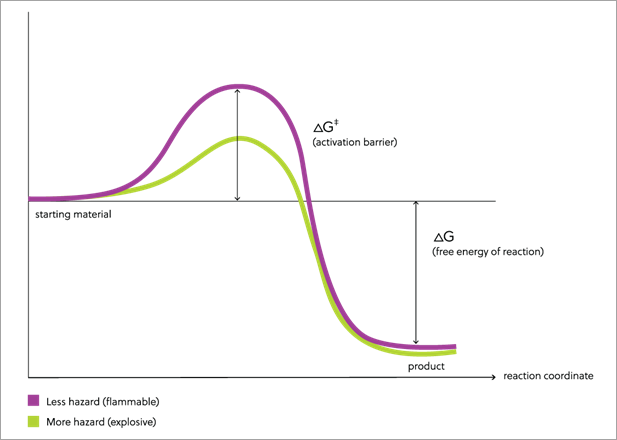
Several processes, additives, and alternatives to ammonium nitrate have been tested in attempts to minimize the dangers of accidental explosions as well as general misuse (Table 1). Despite this, no perfect solution exists, and more work is needed to develop a safe and affordable alternative. Produced by CAS, an Insight Report exploring lessons learned from ammonium nitrate explosions emphasizes that “it is not enough to make fertilizers that do not accidentally explode; it is also important to make ones that cannot be easily made to explode.”
Table 1. List of alternative nitrogenous fertilizers
|
Fertilizer |
Comment |
|---|---|
|
Anhydrous ammonia |
Pressurized gas, Risk Management Plan (RMP)-regulated substance with a threshold of 10,000 lbs, regulated as Dangerous Goods for transportation. |
|
Aqua ammonia |
Volatile, RMP-regulated substance with a threshold of 20,000 lbs |
|
Urea |
High nitrogen content, volatile |
|
Ammonium sulfate |
Non-volatile, low nitrogen content |
|
Diammonium phosphate |
Contains phosphorus |
|
Monoammonium phosphate |
Contains phosphorus |
|
Potassium nitrate |
Contains potassium, stable |
|
Sodium nitrate |
Stable |
|
Calcium cyanamide |
Contains calcium |
|
Calcium nitrate |
Contains calcium |
Alternative nitrogenous fertilizers have also been subject to consideration (Table 1). However, the alternative with the highest nitrogen content is a gas at room temperature, and toxicity prohibits its use. Mixing fertilizers that are high in nitrogen together with other macronutrients can produce effective fertilizer while reducing the risk of explosion.
Handling ammonium nitrate with care
Many stringent regulations and requirements for the safe handling and storage of ammonium nitrate already exist in several countries. In the US, the primary regulations were issued in 2001 by the Occupational Health and Safety Administration (OSHA), and additional guidance can be found in an advisory document issued in 2015 by OSHA in collaboration with the Environmental Protection Agency (EPA), and the Bureau of Alcohol, Tobacco, Firearms, and Explosives (ATF). This advisory was issued as part of an ongoing government initiative to improve ammonium nitrate risk management and safety, as well as to help protect the environment.
The CAS Insight Report on ammonium nitrate safety highlights that its safe storage requires careful consideration of several variables (Figure 2). OSHA regulations stipulate that adequate ventilation is required in storage areas, which is key to preventing toxic gases and hot gases from accumulating as well as agglomeration. Other key safety factors accounted for in legislation include using non-combustible materials in storage areas, keeping temperatures below 130°F, limiting the amount of ammonium nitrate stored in one place, and ensuring adequate firefighting measures.
Legislation and guidelines are vital for the control of a dangerous substance like solid ammonium nitrate, but they can only improve safety if they are adhered to. Improving awareness of the dangers of ammonium nitrate and the importance of following existing guidelines would help prevent or at least greatly limit future catastrophic events.
Ammonium nitrate is a highly useful and economically important substance relied upon for agriculture and other industries throughout the world. However, the dangers of manufacturing and storing it have been highlighted by several devastating explosions over the last century. To improve this dangerous situation, it is paramount that the public is aware of the dangers of ammonium nitrate, and that safety measures are adhered to while suitable alternatives are sought.
If you would like to find out more, please download our Insight Report, “Ammonium Nitrate Explosions: Lessons Learned.”